Abstract
Oxidative stress contributes to dopaminergic neuron degeneration in Parkinson’s disease (PD).Urate, a potent antioxidant, could be neuroprotective. To determine whether higher plasma concentrations of urate predict a reduced risk of PD, we conducted a nested case-control study among participants in the Health Professional Follow-up Study, a cohort comprising over 18,000 men who provided blood samples in 1993–95. 84 incident cases of PD were diagnosed through 2000 and each was randomly matched to two controls by year of birth, race, and time of blood collection. Rate ratios (RR) of PD according to quartile of uricemia were estimated using conditional logistic regression. The mean urate concentration was 5.7mg/dL among cases and 6.1mg/dL among controls (p=0.01). After adjusting for age, smoking, and caffeine, the RR (95 percent confidence interval [CI]) of PD for the highest quartile of uricemia compared to the lowest was 0.43 (0.18, 1.02; p-for-trend=0.017). This association was stronger in analyses excluding cases diagnosed within 4 years (median) from blood collection (RR=0.17; 95 percent CI: 0.04, 0.69; p-for-trend=0.010). These results suggest that high plasma urate concentrations may decrease risk of PD and raise the possibility that interventions to increase plasma urate may reduce risk and delay the progression of PD.
Keywords: Epidemiology, prospective studies, Parkinson’s disease, urate
Plasma urate concentrations are low in most mammals, due to its degradation by uricase. Several mutations in the gene encoding uricase during evolution, however, have resulted in the complete loss of a functional enzyme in apes and humans and a several fold increase in uricemia. It has been proposed that this loss may reflect a beneficial effect of urate against aging, related to its ability to prevent the oxidative damage caused by reactive nitrogen and oxygen species.(1) Because oxidative stress appears to play a key role in the progressive loss of dopaminergic neurons in the substantia nigra that characterizes Parkinson’s disease (PD),(2) urate could be an important determinant of disease susceptibility. The association between urate and risk of PD has been investigated in only two previous prospective studies; in both, there was a trend suggesting that individuals with high serum urate have a lower risk of PD, but these associations were either not significant or only marginally significant(3, 4) and whether urate predicts PD risk remains uncertain. We have therefore conducted a nested-case control study among participants in the Health Professionals Follow-up Study (HPFS) to examine the a priori hypothesis that high plasma levels of urate predict a reduced risk of developing PD.
MATERIALS AND METHODS
Study population
The HPFS was established in 1986, when 51,529 male health professionals (dentists, optometrists, pharmacists, osteopaths, podiatrists, and veterinarians), aged 40–75 years, responded to a mailed questionnaire that included a comprehensive diet survey, in addition to questions on disease history and life style.(5) Participants are mostly white and of European ancestry. Follow-up questionnaires are mailed to participants every two years to update information on potential risk factors for chronic diseases and to ascertain whether major medical events have occurred. From April 1993 through August 1995, blood samples were collected from 18,018 participants in the HPFS cohort. Participants were followed for incidence of PD until the return of the 2002 questionnaire. Those with history of cancer or PD before blood collection were excluded from the study. For each confirmed case of PD, we randomly selected two controls among men who had no report of PD and were alive at the time of the PD diagnosis of their matched case. The controls were matched on year of birth, race (white/other), fasting status at blood draw (> 8 hours vs. less or unknown), time of day of the blood draw in two-hour intervals, and month and year of blood draw. The research herein was approved by the Human Subjects Committees of the Brigham and Women’s Hospital, and the Harvard School of Public Health.
Case ascertainment
Ascertainment of the PD cases in this cohort has been described previously.(6) In brief, after obtaining permission, we asked the treating neurologist of cohort participants who reported a new diagnosis of PD (or internist if the neurologist did not respond) to complete a questionnaire to confirm the diagnosis of PD and the certainty of the diagnosis, or to send a copy of the medical record. A case was confirmed if a diagnosis of PD was considered definite or probable by the treating neurologist or internist, or if the medical record included either a final diagnosis of PD made by a neurologist, or evidence at a neurological assessment of at least two of the four cardinal signs of PD (with one being rest tremor or bradykinesia), a progressive course, and the absence of unresponsiveness to L-dopa or other features suggesting an alternative diagnoses. The review of medical records was conducted by the investigators, blind to the exposure status. Overall, the diagnosis was confirmed by the treating neurologist in 82 percent of the cases, by review of the medical records in 6 percent, and the rest by the treating internist without further support. Because the diagnosis of idiopathic PD remains essentially clinical, the certainty of diagnosis was left to the judgment of the clinician, which has been found to be highly reliable.(7) Deaths in the cohort were reported by family members, coworkers or postal authorities, or were identified by searching the National Death Index. If PD was listed as a cause of death on the death certificate, we requested permission from the family to contact the treating neurologist or physician and followed the same procedure as for the non-fatal cases.
Assessment of exposure
The blood samples were returned to our laboratory via overnight courier; over 95 percent of the samples arrived within 24 hours of being drawn. Upon arrival in our laboratory, the blood samples were centrifuged and blood components aliquotted into cryotubes and stored in the vapor phase of liquid nitrogen freezers. The concentration of urate was determined using a colorimetric enzyme assay on the Hitachi 911 analyzer (Roche Diagnostics - Indianapolis, IN). The day-to-day variabilities of the assay at concentrations of 4.86, 7.20 and 9.39 mg/dL are 1.3, 1.7 and 1.6 percent, respectively. Because the determination of serum urate was conducted specifically for this study and not as part of a blood chemistry panel, results of other routine chemistry tests are not available in these samples.
Statistical analyses
To account for the matched design of the study, we used conditional logistic regression to estimate odds ratios (OR) of PD according to plasma levels of urate, and their 95 percent confidence intervals (CI). Under the design of this study, the odds ratios from conditional logistic regression estimate the corresponding rate ratios (RR), for this reason we have used the latter term throughout the paper. In the main analyses, we categorized plasma levels of urate into quartiles defined according to the distribution of urate among controls. Tests for trend were conducted by including urate as a continuous variable in the conditional logistic regression models. Comparison of means between cases and controls was done using random effects models to account for correlation within matched sets. Data on additional covariates considered in analyses were taken from responses to the 1992 questionnaire—the most recent survey prior to the period of blood donation—except for dietary data (including vitamin use), which were derived from a semiquantitative food-frequency questionnaire administered in 1990 (6) because dietary data were not collected in 1992, and physical activity, which was from the 1986 questionnaire. These covariates included pack-years of smoking, caffeine intake (mg/day), alcohol intake (g/day), body mass index (kg/m2), physical activity (METs/week), dairy, meat, and fish consumption (servings/day), regular use of aspirin or other non-steroidal anti-inflammatory drug use (≥ 2 per week vs. less), use of thiazide or other diuretics, and history of hypertension or gout. Indicator variables were created for covariates with missing data. In regression models alcohol was split into categories based on g/day (0, 1–9, 10–19, 20–29, 30+), while quartiles or quintiles of other covariates were determined with respect to the distribution among the controls. Interactions between urate concentration and other covariates were explored by including in the conditional logistic regression models the product of urate and the covariate of interest, both as continuous variables. Finally, we estimated a summary RR for one standard deviation increase in urate concentration by combining our results with those of the two previous prospective studies.(3, 4) For this purpose, we estimated the RR corresponding to 1.32 mg/dL increase in urate concentration (corresponding to one standard deviation in our cohort) for each study and obtained pooled estimates by averaging the natural logarithms of the RRs from individual studies, weighted by the inverses of their variances. Because there was no heterogeneity among the risk estimates from three cohorts, the pooled RR estimate and confidence interval were estimated using a fixed effect model. The meta-analysis was performed using STATA software version 9 (STATA Corp, College Station, Texas). All other analyses were conducted with the SAS software version 9 (SAS Institute, Cary, North Carolina). All p values are two-sided.
RESULTS
We identified 84 incident cases of PD diagnosed after the time of blood collection. The mean age at PD diagnosis was 71.5 years (range 55 to 85). The mean plasma urate concentration was 5.7 (339 microM/L) among cases and 6.1 mg/dL (363 microM/L) among the 165 matched controls (p=0.0097). Table 1 shows the distribution of covariates among controls by quartile of urate concentration. As expected, body mass index and pack-years of smoking increased somewhat with increasing urate concentration, as did the prevalence of thiazide use, and reported history of high blood pressure or gout. Alcohol consumption increased with each quartile of urate concentration up to the third quartile, but then declined, suggesting that men with high plasma urate refrained from alcohol consumption.
Table 1.
Baseline (1992) characteristics* of controls by urate concentration quartiles, Health Professionals Follow-up Study.
| Urate concentration, mg/dL (micromol/L)
|
||||
|---|---|---|---|---|
| <5.3 (<309) | 5.3–6.0 (310–352) | 6.1–6.9 (352–410) | 7.0–9.7 (411–577) | |
| N | 41 | 42 | 39 | 43 |
| Age at blood collection (1993–95) | 67.8 | 66.2 | 65.8 | 68.7 |
| Body mass index | 24.7 | 23.3 | 26.3 | 26.9 |
| Pack years of smoking | 12.0 | 10.6 | 12.7 | 15.9 |
| Caffeine intake (mg/day) | 227.6 | 202.8 | 227.7 | 231.7 |
| Total alcohol intake (g/day) | 9.3 | 11.7 | 15.3 | 7.6 |
| Alcohol from liquor (g/day) | 4.3 | 4.9 | 7.6 | 4.2 |
| beer (g/day) | 2.9 | 2.4 | 4.1 | 1.5 |
| wine (g/day) | 1.7 | 3.8 | 3.1 | 1.4 |
| Dairy (servings/day) | 2.2 | 1.9 | 2.1 | 1.6 |
| Meat as main dish (servings/day) | 1.14 | 1.07 | 1.29 | 1.04 |
| Fish (servings/day) | 0.38 | 0.40 | 0.32 | 0.29 |
| Physical activity (mets/week) | 23.4 | 21.0 | 19.1 | 18.5 |
| Thiazide users % | 0 | 4.2 | 4.0 | 9.3 |
| Other diuretics % | 0 | 0 | 0 | 1.8 |
| Aspirin users (2+ times/week) % | 43.9 | 38.2 | 45.8 | 37.7 |
| Other NSAIDS % | 13.5 | 6.0 | 7.0 | 9.4 |
| Total NSAIDS % | 45.9 | 38.2 | 48.3 | 37.7 |
| History of high blood pressure % | 15.6 | 26.7 | 32.0 | 48.3 |
| History of self reported gout % | 0 | 6.7 | 12.8 | 18.5 |
Abbreviation: NSAIDS: non-steroidal anti-inflammatory drugs.
All variables except age are age-adjusted by direct standardization to all controls.
Dietary data (including vitamin use) are from 1990.
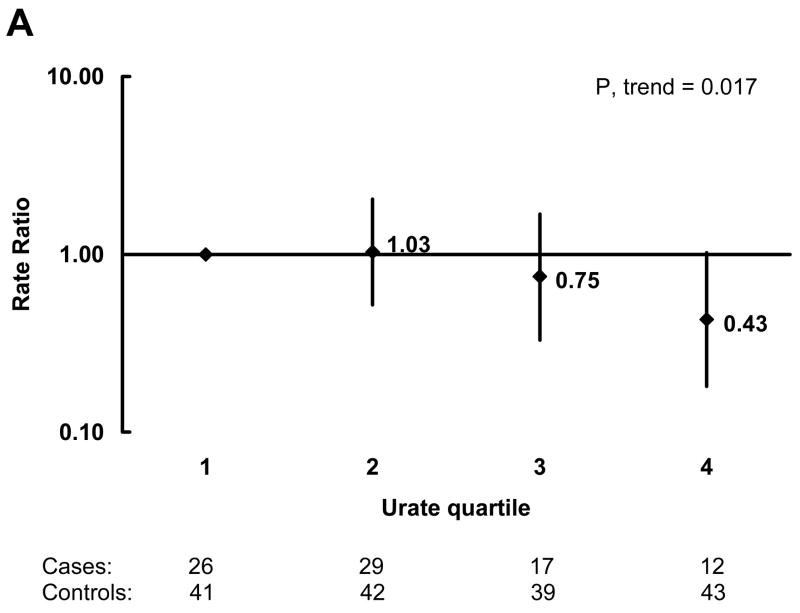
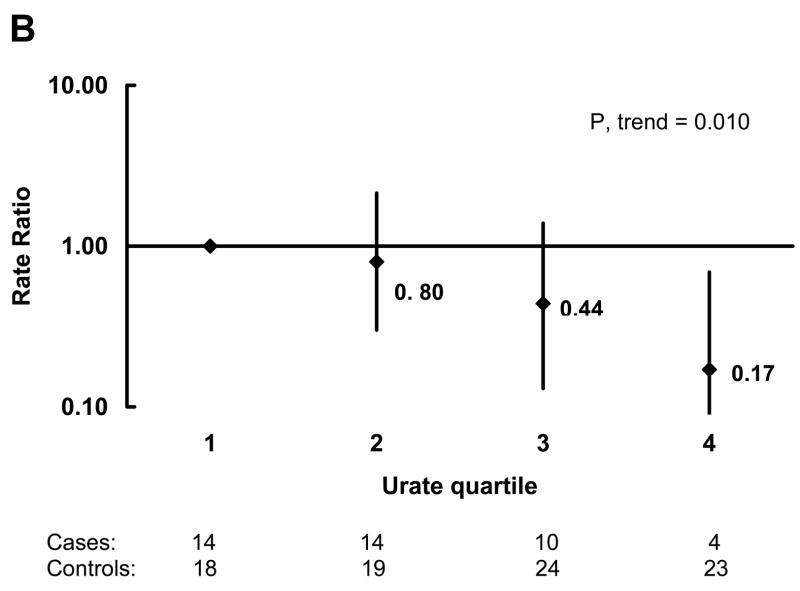
In analyses adjusted for age, pack-years of smoking, and quintiles of caffeine intake, the OR for PD decreased with increasing urate quartile (Figure 1A). The OR (95 percent CI) for the highest quartile compared to the lowest was 0.43 (0.18, 1.02). There was also a significant dose-response relation when urate was analyzed as a continuous variable. The OR (95 percent CI) for PD per unit (mg/dL) increase in urate concentration was 0.76 (0.61, 0.95; p=0.017). In order to reduce the possibility that unrecognized PD could affect urate concentrations, we also did analyses restricted to cases whose blood was drawn more than 4 years (the median) prior to the diagnosis of PD and their matched controls. The association was stronger in these analyses (Figure 1B). The OR (95 percent CI) for the highest quartile compared to the lowest was 0.17 (0.04, 0.69; p=0.013), and the OR (95 percent CI) for PD per unit (mg/dL) increase in urate concentration was 0.62 (0.43, 0.89; p=0.010). Neither history of hypertension nor use of thiazide diuretics were significantly associated with risk of PD, and the association between plasma urate and PD risk was virtually unchanged after adjustment for these variables. Further, the results were not materially affected when the analyses were additionally adjusted for alcohol intake, quintiles of physical activity and body mass index, quartiles of dairy consumption, quintiles of total protein intake, and regular use of non-steroidal anti-inflammatory drug use (yes/no) either individually or simultaneously.
Figure 1.
Odds Ratios (OR) of Parkinson’s disease (PD) by quartile of urate concentration among controls adjusted for age, pack-years of smoking, and quintile of caffeine intake. OR are indicated next to the black diamonds and 95% confidence intervals are indicated by the vertical lines. A) All cases and controls, and B) Only cases whose blood was drawn more than 4 years before their PD diagnosis and their matched controls. The number of cases and controls in each quartile of urate concentration are shown below the figures.
A similar pattern of results was seen when analyses were run based on specific cutoff points of urate concentration. Considering urate concentrations (all in mg/dL) of < 5, 5 to < 5.5, 5.5 to < 6, 6 to < 6.5, 6.5 to < 7, and 7 or higher the number of cases/controls were 22/27, 10/22, 19/30, 11/23, 10/20, and 12/43 in each of those ranges, respectively. The OR (95 percent CI) for PD for those with urate greater than 7 mg/dL compared to those with urate < 5 mg/dL was 0.38 (0.15, 0.96) adjusting for age, pack-years of smoking and caffeine. In the analysis restricted to cases whose blood was drawn more than four years prior to the diagnosis of PD, the number of cases/controls were 10/10, 8/11, 8/14, 7/14, 5/12, 4/23 in the respective categories. The OR (95 percent CI) for PD for those with urate greater than 7 mg/dL compared to those with urate < 5 mg/dL was 0.15 (0.03, 0.63) adjusting for age pack-years of smoking and caffeine.
We also explored the possibility of interactions between urate concentration and other covariates. No significant interactions were found with age at blood collection, pack-years of smoking, caffeine, or alcohol.
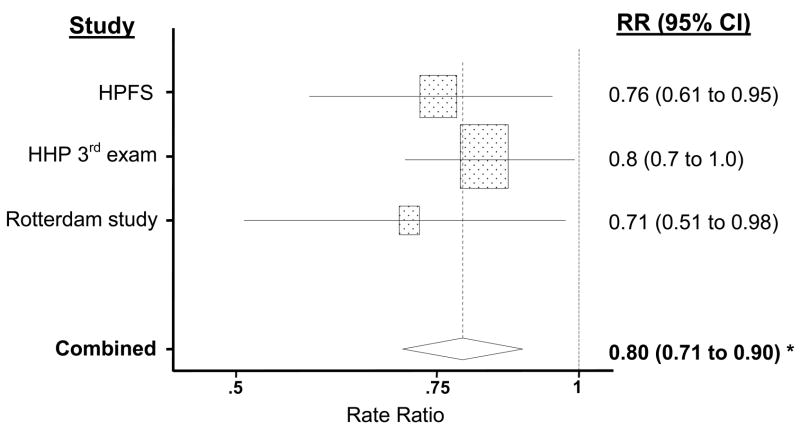
In the meta-analysis of the results of the present study and those of the two previous prospective investigations, the pooled RR of PD associated with a standard deviation increase in urate (1.32 mg/dL) was 0.80 (p=0.000074) (figure 2).
Figure 2.
A meta-analysis of cohort studies of Parkinson’s disease (PD). Rectangles indicate rate ratio ([RR] as estimated by the odds ratio in the present study) of PD corresponding to a 1.32 mg/dL (one standard deviation in the Health Professionals Follow-up Study [HPFS]) increase in the plasma concentration of urate in each study. The size of the rectangle is proportional to the percent weight of the corresponding RR in the meta-analysis; horizontal lines, representing the 95% CI, are plotted on a log scale. The pooled (combined) RR and 95% CI are indicated by the diamond. Urate in the Honolulu Heart Program (HHP) was determined twice, at the time of the first examination—about 30 years before the end of the follow-up—and at the third examination, six years after the first. Results in the figure are based on the third examination because results from the first examination are more likely to be attenuated by unaccounted changes in plasma urate during the follow-up. The pooled RR using results from the first examination would be 0.82 (95% CI: 0.71, 0.95; p = 0.0059). * p<0.0001.
DISCUSSION
In this large prospective investigation we found that men in the top quartile of plasma urate concentration had a 55 percent lower odds of PD than men in the bottom quartile. The decrease in PD odds among men with high levels of urate was stronger among men with blood collected at least four years before the diagnosis of PD, suggesting that the low plasma urate among individuals with PD precedes the onset of neurological symptoms and is thus unlikely to be a consequence of changes in diet, behavior, or medical treatment early in the course of the disease. Further, this inverse association was independent from age, smoking, caffeine consumption and other aspects of lifestyle that have been related to both PD and uricemia.
The difference in plasma urate concentration between cases and controls in our study is unlikely to be artifactual, because both groups were drawn from the same population, and their plasma samples were collected at the same time, processed and stored in the same manner, and sent in random order and without disease status identification for laboratory analyses. A limitation of the present study is that we could not physically examine the participants, and therefore we relied on diagnoses of PD made by the treating neurologists or confirmed by review of the medical records. Although some diagnostic misclassification may have occurred, error from this source is probably modest because according to recent clinico-pathological studies the accuracy of the clinical diagnosis of PD made by neurologists is around 90 percent.(7) Most importantly, diagnostic errors are probably unrelated to plasma urate concentration, and would thus tend to attenuate any true association. An additional limitation is that we relied on a single measurement of plasma urate. Repeated blood samples over time would allow a more accurate determination of long term average urate concentration, which would be expected a priori to be the strongest predictor of PD. Because the error in assessing average urate concentration is most likely independent from odds of PD, it would also tend to attenuate the association between urate and odds of PD.
Direct evidence from studies in humans of a role for urate in the development of PD has so far been modest. Urate was found to be reduced in serum,(8) cerebrospinal fluid,(9) and post-mortem substantia nigra(10) of patients with PD as compared with controls, but these findings could have been consequences rather than causes of the disease process. In the Honolulu Heart Program, which included about 8,000 men of Japanese ancestry followed for 30 years, the age- and smoking-adjusted rate of PD was 40 percent lower among men with serum urate above the median as compared with those with urate below the median.(3) The overall trend suggest a 20–30 percent decrease in rate of PD for each standard deviation increase in urate concentration, but the statistical significance for an association was marginal. A similar association was observed in the Rotterdam study, comprising 4,695 men and women and 68 incident cases of PD during an average of nine years of follow-up.(4) The consistency between the rate ratio estimates in these previous studies and that presented here is impressive, and the pooled results provide compelling evidence of a decreasing rate of PD for higher levels of urate.
Because of its observational design, the results of the present and previous investigations cannot establish whether high levels of urate are causally related to rate of PD, or whether uricemia and low rate of PD share an unknown common cause. A preventive effect of uricemia, however, would be consistent with the strong evidence for a role of oxidative stress in the progressive degeneration of dopaminergic neurons in individuals with PD.(2, 11) The oxidative metabolism of dopamine can produce hydrogen peroxide and other reactive oxygen species, and conditions favorable to oxidative stress, including increased iron,(12, 13) decreased glutathione, and signs of oxidative damage to lipids, proteins, and DNA have been observed in postmortem PD brains.(11) Urate is present in plasma as the sodium salt, at high concentrations maintained by active kidney reabsorption.(1, 14) Urate in physiological concentrations is as effective as ascorbate in preventing lipid peroxidation initiated in vitro by hydrogen peroxide;(1) stabilizes ascorbate,(15) possibly by forming complexes with iron ions;(16) and scavenges nitrogen radicals,(17, 18) which have a critical role in MPTP-induced dopaminergic neurotoxicity, an animal model of PD.(19, 20) Further, administration of urate reduced the homocysteine-induced exacerbation of the oxidative stress and mitochondrial dysfunction in human dopaminergic cells exposed to the pesticides rotenone or to iron ions.(21)
Although cerebrospinal fluid urate concentration is only about 7 percent that of plasma, there is a strong correlation between plasma and cerebrospinal fluid urate,(22) and, in spite of being present at low concentrations, urate could still be important in the brain because of its distinct antioxidant effects(23) or its ability to stabilize ascorbate. It is also possible that a localized increase in the blood-brain barrier permeability precedes the clinical onset of PD. Changes in blood vessels in the substantia nigra of PD patients have been described,(24) and the results of a recent study provide preliminary evidence of a blood-brain barrier dysfunction.(25) Urate seems to preventthe increase in permeability of the blood brain barrier observed in inflammatory diseases of the central nervous system, such as Borna disease virus encephalitis(26) or experimental allergic encephalomyelitis (EAE), an animal model of multiple sclerosis.(27–30) Consistently with the results in EAE, low urate has been associated with optic neuritis(31) and multiple sclerosis.(32–34)
The hypothesis that uricemia could effectively reduce the susceptibility to PD has potentially important therapeutic implications because plasma levels of urate can be increased by inosine, a urate precursor sold over the counter as a nutritional supplement and athletic performance enhancer,(35) which is now being tested in a randomized trial for multiple sclerosis treatment.(36) A primary prevention trial for PD would be unfeasible, because elevated levels of urate are an important risk factor for cardiovascular diseases and overall mortality. In the Honolulu Heart Program cohort itself, men in the top quartile of serum urate concentration had a reduced rate of PD, but a 20 percent increased total mortality rate. Whether uricemia itself is harmful or whether it is a marker of an underlying systemic stress remains to be established, but, if the harm is real, any putative beneficial effect of increasing plasma urate concentration on risk of PD would be offset by increased cardiovascular morbidity and mortality. A more cogent question, therefore, is whether increasing plasma urate concentration could decelerate the progression of neurodegeneration among individuals with PD, for whom the benefits of neuroprotection could possibly outweigh the potential adverse effects. The recent findings of a slower rate of PD progression among individuals with higher serum urate(37) or cerebrospinal fluid urate(38) concentrations support this possibility.
Acknowledgments
The study was partly funded by NIH/NINDS grant R01 NS048517 and a gift from the Kinetics Foundation, and was supported in part by the Intramural Research Program of the NIH, and NIEHS. Dr. Weisskopf is the recipient of NIEHS grant K01 ES012653.
Abbreviations
- CI
confidence interval
- EAE
experimental allergic encephalomyelitis
- HPFS
Health Professionals Follow-up Cohort
- OR
odds ratio
- PD
Parkinson’s disease
- RR
rate ratio
References
- 1.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78:6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36–8. [DOI] [PubMed] [Google Scholar]
- 3.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid and the risk of idiopathic Parkinson’s disease. Am J Epidemiol. 1996;144:480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 4.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58:797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 5.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 6.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 7.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57:1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 8.Larumbe Ilundain R, Ferrer Valls JV, Vines Rueda JJ, Guerrero D, Fraile P. [Case-control study of markers of oxidative stress and metabolism of blood iron in Parkinson’s disease] Rev Esp Salud Publica. 2001;75:43–53. [PubMed] [Google Scholar]
- 9.Tohgi H, Abe T, Takahashi S, Kikuchi T. The urate and xanthine concentrations in the cerebrospinal fluid in patients with vascular dementia of the Binswanger type, Alzheimer type dementia, and Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1993;6:119–26. doi: 10.1007/BF02261005. [DOI] [PubMed] [Google Scholar]
- 10.Church WH, Ward VL. Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res Bull. 1994;33:419–25. doi: 10.1016/0361-9230(94)90285-2. [DOI] [PubMed] [Google Scholar]
- 11.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–44. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Sofic E, Paulus W, Jellinger K, Riederer P, Youdim MBH. Selective Increase of Iron in Substantia Nigra Zona Compacta of Parkinisonian Brains. Journal of Neurochemistry. 1991;56:978–982. doi: 10.1111/j.1471-4159.1991.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 13.Dexter DT, Wells FR, Lees AJ, et al. Increased Nigral Iron Content and Alterations in Other Metal Ions Occurring in Brain in Parkinson’s Disease. Journal of Neurochemistry. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 14.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–52. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 15.Stocker R, Frei B. Endogenous antioxidant defences in human blood plasma. 1991:213–243. [Google Scholar]
- 16.Davies KJ, Sevanian A, Muakkassah-Kelly SF, Hochstein P. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235:747–54. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squadrito GL, Cueto R, Splenser AE, et al. Reaction of uric acid with peroxynitrite and implications for the mechanism of neuroprotection by uric acid. Arch Biochem Biophys. 2000;376:333–7. doi: 10.1006/abbi.2000.1721. [DOI] [PubMed] [Google Scholar]
- 18.Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann N Y Acad Sci. 2002;962:242–59. doi: 10.1111/j.1749-6632.2002.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 19.Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci U S A. 1996;93:4565–71. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberatore GT, Jackson-Lewis V, Vukosavic S, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–9. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 21.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002;80:101–10. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 22.Niklasson F, Agren H. Brain energy metabolism and blood-brain barrier permeability in depressive patients: analyses of creatine, creatinine, urate, and albumin in CSF and blood. Biol Psychiatry. 1984;19:1183–206. [PubMed] [Google Scholar]
- 23.Anderson RF, Harris TA. Dopamine and uric acid act as antioxidants in the repair of DNA radicals: implications in Parkinson’s disease. Free Radic Res. 2003;37:1131–6. doi: 10.1080/10715760310001604134. [DOI] [PubMed] [Google Scholar]
- 24.Faucheux BA, Bonnet AM, Agid Y, Hirsch EC. Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet. 1999;353:981–2. doi: 10.1016/S0140-6736(99)00641-8. [DOI] [PubMed] [Google Scholar]
- 25.Kortekaas R, Leenders KL, van Oostrom JC, et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–9. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 26.Hooper DC, Kean RB, Scott GS, et al. The central nervous system inflammatory response to neurotropic virus infection is peroxynitrite dependent. J Immunol. 2001;167:3470–7. doi: 10.4049/jimmunol.167.6.3470. [DOI] [PubMed] [Google Scholar]
- 27.Spitsin SV, Scott GS, Mikheeva T, et al. Comparison of uric acid and ascorbic acid in protection against EAE. Free Radic Biol Med. 2002;33:1363–71. doi: 10.1016/s0891-5849(02)01048-1. [DOI] [PubMed] [Google Scholar]
- 28.Hooper DC, Spitsin S, Kean RB, et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci U S A. 1998;95:675–80. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott GS, Spitsin SV, Kean RB, Mikheeva T, Koprowski H, Hooper DC. Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc Natl Acad Sci U S A. 2002;99:16303–8. doi: 10.1073/pnas.212645999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott GS, Hooper DC. The role of uric acid in protection against peroxynitrite-mediated pathology. Med Hypotheses. 2001;56:95–100. doi: 10.1054/mehy.2000.1118. [DOI] [PubMed] [Google Scholar]
- 31.Knapp CM, Constantinescu CS, Tan JH, McLean R, Cherryman GR, Gottlob I. Serum uric acid levels in optic neuritis. Mult Scler. 2004;10:278–80. doi: 10.1191/1352458504ms1042oa. [DOI] [PubMed] [Google Scholar]
- 32.Spitsin S, Hooper DC, Mikheeva T, Koprowski H. Uric acid levels in patients with multiple sclerosis: analysis in mono- and dizygotic twins. Mult Scler. 2001;7:165–6. doi: 10.1177/135245850100700305. [DOI] [PubMed] [Google Scholar]
- 33.Toncev G, Milicic B, Toncev S, Samardzic G. Serum uric acid levels in multiple sclerosis patients correlate with activity of disease and blood-brain barrier dysfunction. Eur J Neurol. 2002;9:221–6. doi: 10.1046/j.1468-1331.2002.00384.x. [DOI] [PubMed] [Google Scholar]
- 34.Sotgiu S, Pugliatti M, Sanna A, et al. Serum uric acid and multiple sclerosis. Neurol Sci. 2002;23:183–8. doi: 10.1007/s100720200059. [DOI] [PubMed] [Google Scholar]
- 35.Starling RD, Trappe TA, Short KR, et al. Effect of inosine supplementation on aerobic and anaerobic cycling performance. Med Sci Sports Exerc. 1996;28:1193–8. doi: 10.1097/00005768-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Koprowski H, Spitsin SV, Hooper DC. Prospects for the treatment of multiple sclerosis by raising serum levels of uric acid, a scavenger of peroxynitrite. Ann Neurol. 2001;49:139. doi: 10.1002/1531-8249(200101)49:1<139::aid-ana28>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 37.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate level predicts progression of Parkinson’s disease (Abstract). Society for Neuroscience, 36th Annual Meeting; Atlanta, USA. 2006. Abstract #510.2 [ http://www.sfn.org/am2006] [Google Scholar]
- 38.Ascherio A, LeWitt PA, Watts A, et al. CSF as well as serum urate are predictors of Parkinson’s disease progression. (Abstract). 10th International Conference of Parkinson’s Disease and Movement Disorders; Kyoto, Japan. 2006. p. LB-2. [Google Scholar]