Abstract
Given the growing body of evidence for a role of glia in pain modulation, it is plausible that the exaggerated visceral pain in chronic conditions might be regulated by glial activation. In this study, we have investigated a possible role for microglia in rats with chronic visceral hypersensitivity and previously documented altered neuronal function. Experiments were performed on adult male Sprague-Dawley rats pre-treated with neonatal colon irritation (CI) and on control rats. Effects of fractalkine (FKN, a chemokine involved in neuron-to-microglia signaling) and of minocycline (an inhibitor of microglia) on visceral sensitivity were examined. Visceral sensitivity was assessed by recording the electromyographic (EMG) responses to graded colorectal distension (CRD) in mildly sedated rats. Responses to CRD were recorded before and after injection of FKN, minocycline or vehicle. Somatic thermal hyperalgesia was measured by latency of paw withdrawal to radiant heat. The pattern and intensity of microglial distribution at L6–S2 in the spinal cord was also compared in rats with CI and controls by fluorescence microscopy using OX-42. Results show that: (1) FKN significantly facilitated EMG responses to noxious CRD by >52% in control rats. FKN also induced thermal hyperalgesia in control rats, consistent with previous reports; (2) minocycline significantly inhibited EMG responses to noxious CRD by >70% in rats with CI compared to controls 60 min after injection. The anti-nociceptive effect of minocycline lasted for 180 min in rats with CI, reaching peak values 60 min after injection. Our results show that FKN enhances visceral and somatic nociception, whereas minocycline inhibits visceral hypersensitivity in chronically sensitized rats, which indicates a role for microglia in visceral hypersensitivity.
Keywords: Visceral pain, hypersensitivity, neonatal injury, colon, microglia
INTRODUCTION
Interest in the link between glia and pathological pain has increased greatly in the past couple of decades (DeLeo et al., 2006). However, most reports on the contribution of glia to pain study somatic rather than visceral forms of chronic pain. Historically, glia (microglia, astrocytes and oligodendrocytes) in the CNS have been perceived collectively as the ‘third element’ that simply fills spaces around neurons, the ‘truly’ functional cells of the nervous system (Somjen, 1988). However, it is now widely recognized that glia maintain general homeostasis of the CNS in ways that impact on neuronal function (Kettenmann and Ransom, 2005).
Microglia belong to the mononuclear phagocyte-lineage and are related to other organ-specific macrophages such as Kupffer cells in the liver (Vilhardt, 2005). Of hematopoietic origin, microglia populate the CNS during early development, forming a regularly spaced network of cells that rival the neuronal population in numbers, according to conservative estimates (Colognato and ffrench-Constant, 2004; Kettenmann and Ransom, 2005). Moreover, microglia constitute a first line of defense for the CNS, acting as immune-alert resident macrophages and a rapid sensor of CNS pathology (Kreutzberg, 1996).
Modulators of microglial function, such as fractalkine (FKN or CX3C ligand-1 chemokine) and minocycline, have been administered to rodents in many studies to influence chronic pain behavior. FKN is tethered to the membrane surface of primary afferents within the spinal cord. On traumatic nerve stimulation, FKN is cleaved and released from the central terminals of primary afferents. It then binds to its specific receptor (CX3CR-1) which is expressed predominantly by spinal cord microglia, thus, causing microglial activation (reviewed in Watkins et al., 2001a; Watkins et al., 2001b; Watkins and Maier, 2003; Milligan et al., 2005). Hence, FKN is often considered to be a specific neuron-to-microglia signal. By contrast, minocycline is a second-generation tetracycline that inhibits microglial activation under various pathological conditions without affecting neurons, astroglia, and oligodendroglial progenitors (Raghavendra et al., 2003; Raghavendra et al., 2004).
According to several studies using somatosensory behavioral tests, intrathecal administration of FKN evokes dose-dependent mechanical allodynia and thermal hyperalgesia in naïve rats (Milligan et al., 2004) whereas FKN-induced pain behavior is prevented by minocycline (Milligan et al., 2005). In the spinal cord, concomitant with microglial activation, CX3CR-1 expression is upregulated after chronic constriction injury of the sciatic nerve (Verge et al., 2004). Intrathecal administration of a neutralizing antibody against CX3CR-1 either delays or reduces neuropathic behavior (Milligan et al., 2004). By contrast, intrathecal minocycline attenuates neuropathic behavior one day, but not one week, after neuropathic injury (Ledeboer et al., 2005). Systemic administration of minocycline also prevents the development of neuropathic (Raghavendra et al., 2003) and inflammatory pain (Cho et al., 2006). Recently, our group and colleagues have also used either FKN or minocycline to demonstrate that hyperexcitability of dorsal horn neurons with somatic receptive fields after injury to the sciatic nerve (Owolabi and Saab, 2006) or spinal cord (Hains and Waxman, 2005) is partly attributable to microglial activation.
OBJECTIVE
An association between chronic non-inflammatory visceral pain and microglial function, similar to that which links somatic pain and microglial activation, has not been demonstrated previously. We hypothesized that behavioral manifestation of visceral pain might be influenced by pharmacological modulation of microglial function in control rats and in those with chronic colon hypersensitivity.
METHODS
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences and were in accordance with the guidelines of the National Institutes of Health and the International Association for the Study of Pain. Experiments were performed using male Sprague-Dawley rats obtained as neonates on postnatal day (PND) 6 from Harlan Sprague-Dawley Inc. Rats were housed with their mothers in plastic cages and maintained on a 12:12 hour light:dark cycle. Mothers had access to water and food ad libitum.
Colon irritation
The neonatal colon irritation (CI) model has been described in detail previously (Al-Chaer et al., 2000; Lin and Al-Chaer, 2005). On PND8, PND10 and PND12, rats in one group (the CI group, n = 11) were exposed to colorectal distension using an angioplasty balloon (Advanced Polymers Inc.) inserted through the anus into the descending colon. The balloon was distended using 0.3 ml of water (pressure of 60 mm Hg, measured with a sphygmomanometer) for 1 min and then deflated and withdrawn. This stimulus was applied twice with a 30-min rest between distensions. Naïve rats in another group (control rats, n = 12) were handled similarly to those with CI with the exception of balloon insertion. Instead, these rats were held and gently touched on the perineal area on PND8, PND10 and PND12. At PND25, all rats were weaned and separated into their own cages. No further treatment or procedures were undertaken until rats were 3-months old (adulthood).
Behavioral visceral testing
Visceral hypersensitivity was assessed by recording the electromyographic (EMG) responses to graded colorectal distention (CRD, see below) in rats sedated mildly with isoflurane (1%). The EMG electrode (Teflon-coated silver wire) was inserted into the external oblique muscle superior to the inguinal ligament and connected to an ISO-80 amplifier (WPI). The signal was displayed on a Tektronix 2012 oscilloscope (Richardson), fed into a computer using CED 1401-plus (Cambridge Electronic Design) and recorded using Spike 3 software. CRD was administered as outlined below. To establish a baseline of activity for each individual animal, each rat was tested with two initial CRDs (80 mmHg) for 30 sec separated by a 10-min rest. 10 min after the second priming, a baseline response to the graded CRD was recorded. For analysis, the raw EMG signal was rectified (full-wave) offline using Spike 3 script. The EMG responses were measured following treatment with vehicle, minocycline (6 mg ml−1, i.p.) or FKN (8 µg ml−1, i.t.). The full duration of the behavioral testing did not exceed 70 min for each rat.
CRD
CRD was produced by inflating a balloon inserted through the anus inside the descending colon and rectum. The balloon was 4–6 cm in length (made from the finger of a latex glove), attached to polyethylene tubing. The open end of the balloon was secured to the tubing with thread and wrapped with tape (1 cm wide). The balloon was inserted so the thread was ~1 cm proximal to the anal sphincter and held in place by taping the tubing to the tail. This tubing was attached via a ‘T’ connector to a sphygmomanometer pump and gauge. Before use, the balloon was blown up and left overnight so that the latex would stretch and the balloon become compliant. Distension was produced by rapidly inflating the latex balloon to 20, 40, 60 or 80 mmHg for 20 sec with a 4-min interval between distensions.
Behavioral somatic testing
All paw-withdrawal experiments were performed using a Hargreave’s Apparatus (Ugo Basile, Italy). Rats were placed in a small plastic enclosure and allowed to acclimate for 30 min. Radiant heat from a light source was applied to the plantar surface of the hindpaw. Animals were free to escape by withdrawing their hindpaws away from noxious stimuli, and the paw-withdrawal latency (PWL) was measured. Thermal hyperalgesia was indicated by a reduction in PWL. Each hindpaw was tested three times, and the animal allowed to rest for 20 min between tests. Animals were tested 40 min after injection of FKN.
Immunohistochemistry
Tissue was collected from L6–S2 regions of the spinal cord. Rats were anesthetized deeply with isoflurane and perfused intracardially with 0.9% saline. The tissue was fixed with 4% paraformaldehyde overnight at 4°C then transferred to a 30% sucrose PBS solution for 1–2 days. Thin (9 µm) cryosections were processed simultaneously for detection of microglia. Slices were placed in a blocking solution (PBS containing 5% NGS, 2% BSA, 0.1% Triton X-100 and 0.02% sodium azide) for 30 min at room temperature, then incubated with mouse anti-rat CD11b/c OX-42 (1:500, BD Biosciences) in blocking solution overnight at 4°C. Slices were then washed with PBS six times for 5 min per wash, and incubated with Alexa Fluor® 546 goat anti-mouse IgG (H+L) (1:1000, Invitrogen) for 2 hours at room temperature. After a final wash (PBS six times for 5 min per wash) the slices were mounted on slides using Vectashield mounting medium (Vector Laboratories Inc.). OX-42 immunofluorescence was estimated by computer-assisted image analysis. Images of the dorsal quadrant of the spinal cord were acquired using a Nikon microscope with a 10 X objective and analyzed with MetaMorph software (Molecular Devices Corp.). An image threshold was manually set when microglial profiles were readily distinguished from background, calibrated for 10 X distances, and percent area was determined. The same square area and threshold were used for tissue sections from rats with CI and control.
Intrathecal administration
Rats were anesthetized with isoflurane (2%). An intrathecal catheter (PE-10, 7.5-cm long) was inserted caudally into the inter-vertebral gap between L4 and L5 and extended to the subarachnoid space of the spinal lumbar enlargement. The catheter was filled initially with PBS. FKN was injected intrathecally in a 5 µl volume followed by 10 µl of PBS to flush the catheter. In all manipulations, behavioral and histological, the investigator was blinded to the type of rats.
Statistical analysis was done using SigmaStat. The data were compared point to point using a one-way ANOVA followed by t-test (data are shown ± S.D.).
RESULTS
This study demonstrates that FKN induces behavioral sensitization to visceral stimulation, whereas minocycline attenuates existing visceral hypersensitivity in a model of CI associated with microglial activation. All rats exhibited behavioral manifestation of visceral hypersensitivity following FKN administration. Responses to graded CRD (40–80 mmHg) measured by EMG activity increased in control rats 20 min after FKN injection compared with responses recorded in rats after injection of an equal volume of vehicle (Fig. 1A). Significant increases in the average responses to CRD were observed at 60 and 80 mmHg (1.3 ± 0.6 and 3.4 ± 2.3 after FKN compared to 0.1 ± 0.1 and 1.6 ± 1.7 after vehicle, respectively, n = 5) (Fig. 1B). In support of a previous report describing thermal hyperalgesia after FKN treatment (Milligan et al., 2004), we confirm comparable results that further corroborate our experimental paradigm: PWL decreased significantly in right (10.8 ± 0.8) and left (9.8 ± 0.9) hindpaws following FKN treatment compared to vehicle (13.5 ± 2.0 and 13.6 ± 2.7, respectively) (Fig. 1C). By contrast, systemic minocycline caused no change in baseline EMG responses to CRD within 1–3 hours of administration in control rats (Fig. 2, n = 4).
Fig. 1.
(A) Sample waveform traces illustrate EMG responses to CRD under light anesthesia during graded distensions of the balloon to 20, 40, 60 and 80 mmHg. Each CRD was applied for 20 sec (top horizontal bars). Note enhanced responses to stimuli in the range of 40–80 mmHg in a control rat within 20 min after i.t. injection of FKN (right) compared to another rat after vehicle (left). (B) Average EMG responses in control rats injected with either FKN or vehicle. Significant increases were seen in response to CRD intensities of 60 and 80 mmHg after FKN injection compared to vehicle. (C) The decrease in paw withdrawal latencies recorded in the hindpaws of rats after FKN injection confirms thermal hyperalgesia (**P<0.01, *P<0.05).
Fig. 2. Average EMG responses to CRD in control rats treated with vehicle or minocycline.
No significant changes were observed after 1, 2 or 3 hours treatment with minocycline.
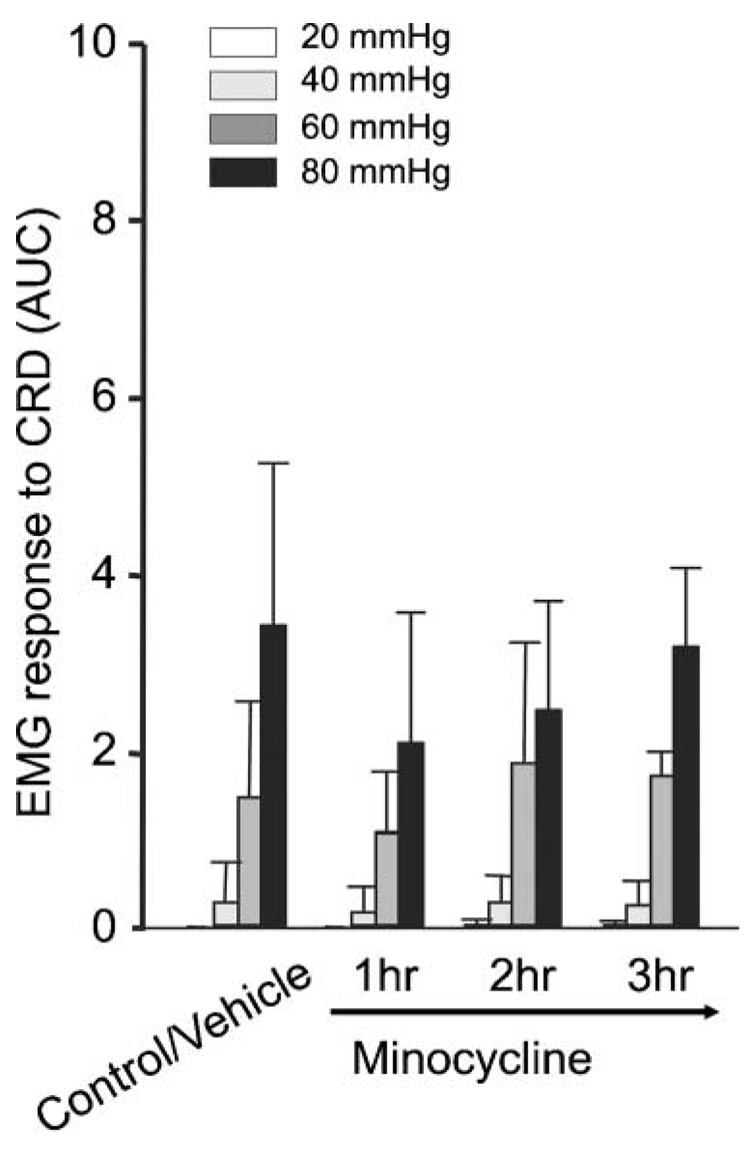
In rats with CI, OX-42 immunohistochemistry revealed a more intense staining pattern compared to controls and an increased number of labeled microglial profiles, which indicates microglial activation under neonatal CI conditions. Representative sections from L6–S2 (Fig. 3A) correspond to the spinal levels where pelvic visceral input converges onto the spinal cord (Al-Chaer and Traub, 2002). As documented previously (Al-Chaer et al., 2000), rats with CI manifest increased responses to CRD than controls. Minocycline attenuated these responses significantly in rats with CI (Fig. 3B, compare to traces in Fig. 1A). Within 1 hour after minocycline treatment the average responses to CRD at 40, 60 and 80 mmHg were reduced significantly (0.1 ± 0.1, 0.6 ± 0.3 and 1.5 ± 0.3 after minocycline compared to 0.4 ± 0.3, 2.9 ± 1.1 and 5.6 ± 2.0 after vehicle, respectively, n = 4) (Fig. 3C). Significant reduction in EMG responses to CRD were still observed in the noxious range (80 mmHg) at 2 hours and 3 hours after minocycline, indicating reversibility of the anti-nociceptive effects within 1 hour of treatment in response to less noxious or non-noxious CRD (20–60 mmHg). In rats with CI, EMG responses to CRD were enhanced further following FKN administration. In particular, responses to CRD at 80 mmHg increased significantly, from 2.1 ± 0.4 to 4.0 ± 0.5 (Fig. 4, n = 5), indicating the possibility for a more heightened state of visceral hypersensitivity in rats with CI.
Fig. 3.
(A) Photomicrographs of spinal cord cross-sections at L6 reveal prominent OX-42 fluorescence in rats with CI compared to control, indicating activated microglia (dotted circles guide to the region of the central canal). Right: estimated relative OX-42 intensity. (B) Sample waveforms illustrate EMG responses to CRD in a rat with CI treated with vehicle (left) or to that in another rat with CI after minocycline (right). Minocycline attenuates the EMG responses to CRD. (C) EMG responses to CRD in rats CI treated with either minocycline or vehicle. Minocycline significantly reduced the average responses to CRD intensities of 40, 60 and 80 mmHg 1 hour after injection. The effect on the response to CRD at 80 mmHg lasted up to 3 hours (**P<0.01, *P<0.05).
Fig. 4. Average EMG responses to CRD in rats with CI treated with either vehicle or FKN.
FKN injection significantly enhanced the average EMG response to CRD at 80 mmHg (*P<0.05).
CONCLUSIONS
In this study, we tested our hypothesis that FKN (an activator of microglia) induces behavioral sensitization to visceral stimulation, whereas minocycline (an inhibitor of microglia) attenuates existing visceral hypersensitivity in adult rats with neonatal CI associated with central sensitization.
DISCUSSION
Our findings support existing literature regarding microglia and somatic pain (DeLeo and Yezierski, 2001; Watkins and Maier, 2002; Watkins and Maier, 2003) and a recent study describing upregulation of glial fibrillary acidic protein in astrocytes correlated in time with colonic inflammation and in space with neuronal increase of Fos expression (Sun et al., 2005).
Following neonatal CI adult rats exhibit visceral hypersensitivity associated with central neuronal sensitization (Al-Chaer et al., 2000) and sensitization of primary afferents innervating the colon (Lin and Al-Chaer, 2005). In this study, we have shown that this visceral hypersensitivity is also associated with microglial activation. Our results corroborate those of several other studies showing significant contribution of FKN to neuropathic (albeit somatic) pain behavior (Milligan et al., 2004), and attenuation of pain behavior by minocycline (Ledeboer et al., 2005). Microglial activation is triggered by FKN, intradermal formalin injection (Fu et al., 2000), direct exposure to the HIV viral envelop (Milligan et al., 2000), and other types of traumatic nerve stimulation at the corresponding spinal cord level (Graeber and Kreutzberg, 1988; Garrison et al., 1991; Herzberg and Sagen, 2001; Jin et al., 2003) including electric current (Hall et al., 1989), and secondary to injury caused by either axotomy or cryoneurolysis (Graeber and Kreutzberg, 1988; Graeber et al., 1988; Tetzlaff et al., 1988; Hall et al., 1989), chronic constriction (Colburn et al., 1999) and partial ligation (Coyle, 1998). Here, we add CI in the neonatal stage to this list as a novel trigger for microglial activation during adulthood at a spinal cord level that receives sensory input from visceral afferents (Willis et al., 1999; Al-Chaer and Traub, 2002).
Microglial activation is described as the gradual acquisition of multiple functions including tissue repair, phagocytosis of cellular debris and pathogens, induction of inflammation and activation of lymphocytes. On activation, microglia secrete proinflammatory mediators such as prostaglandins, proteases, cytokines such as tumor necrosis factor α, interleukin 1β (IL-1β) and IL-6 and excitatory amino acids (Kettenmann and Ransom, 2005; DeLeo et al., 2006) whose receptors are expressed on neurons in the dorsal horn, in addition to generating reactive oxygen species and nitrogen intermediates that affect the biophysical properties of neurons. The time-frame of the somatic and visceral pro-nociceptive effects (within 20–30 min) that are induced by FKN is in accordance with data reported previously at behavioral (Milligan et al., 2004) and cellular levels (Owolabi and Saab, 2006), which indicates comparable signaling mechanisms might underlie microglia–neuron communication during sensory processing of either somatic or visceral pain information in the spinal cord.
However, one important distinction concerns the efficacy of minocycline in reversing chronic visceral hypersensitivity in adulthood long after the initial neonatal insult. This contrasts, for example, with the failure of minocycline to abrogate somatic neuropathic behavior (Ledeboer et al., 2005) and hyperexcitability of neurons with somatic receptive fields (Owolabi and Saab, 2006) if not administered <1 week after sciatic injury. It is also speculated that microglial activation parallels the initial stages rather than the later stages of somatic neuropathic pain (Tanga et al., 2005). We note, however, that minocycline can restore neuropathic manifestations at behavioral and cellular levels up to 4 weeks after traumatic spinal cord injury (Hains and Waxman, 2006). Furthermore, tactile allodynia is attenuated 10 days after spinal nerve injury by spinal inhibition of p38 mitogen-activated protein kinase (p38MAPK) in microglia, which, consequently, inhibits microglia (Jin et al., 2003). Further experiments are needed to resolve this apparent model-specific incongruity in the time-course of the effect of minocycline, especially if considering clinically suitable treatment options for patients with either CNS or gastrointestinal disorders following trauma or inflammation. Alternatively, the use of anesthetics in different experimental settings might influence glial function (Lockwood et al., 1993). Nonetheless, we speculate that the contribution of microglia to visceral pain in chronic conditions is potentially reversible by minocycline treatment.
We have reported earlier hypersensitivity and neural sensitization (both peripheral and central) in adult rats with CI despite the lack of obvious histological pathology or inflammation of the colon (Al-Chaer et al., 2000). However, it is possible that either bacteria or other antigens might transmigrate or ‘leak’ from the colon to target visceral afferents and cause peripheral neuronal inflammation. Although systemic administration of minocycline has been demonstrated to either prevent or reverse spinal microglial activation in chronic pain conditions (Rhagavendra et al., 2003; Cho et al., 2006), future studies are needed to distinguish between the inhibition by minocycline of spinal microglia and its peripheral anti-inflammatory effects.
Microglia and neurons undergo reciprocal interactions under normal conditions. Interruption of this specific communication is responsible for switching microglia to a hyperactive state (Streit, 2002; Polazzi and Contestabile, 2002). Even in a resting state, microglia are not completely inactive and they interact dynamically with other CNS glia and neurons, fulfilling neurotrophic roles. The mechanisms of microglial activation in rats with CI and those underlying behavioral manifestations are not understood completely. The neonatal timing of the colon injury causes long term visceral sensitization that is sustained by CNS mechanisms (e.g. Saab et al., 2004), so might cause a state of persistent microglial activation, which, in turn, feeds forward the neuronal sensitization.
Last, our data indicate that a CI insult in neonatal rats switches microglia to a long-term hyperactive state, which is likely to affect the physiological microglia-neuron equilibrium in the CNS. Transient interruption in microglial activation (and possibly their release of proinflammatory mediators) using minocycline might reverse visceral pain behavior and restore normal processing of neuronal information along the visceral pain pathway during adulthood.
ACKNOWLEDGEMENTS
This study was supported by NIH R01-NS40434, RR020146 and Rhode Island Foundation. The authors thank Mr Beau Strotman for technical assistance.
REFERENCES
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Al-Chaer ED, Traub RJ. Biological basis of visceral pain: recent developments. Pain. 2002;96:221–225. doi: 10.1016/S0304-3959(02)00046-5. [DOI] [PubMed] [Google Scholar]
- Cho IH, Chung YM, Park CK, Park SH, Li HY, Kim D, et al. Systemic administration of minocycline inhibits formalin-induced inflammatory pain in rat. Brain Research. 2006;1072:208–214. doi: 10.1016/j.brainres.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Experimental Neurology. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- Colognato H, ffrench-Constant C. Mechanisms of glial development. Current Opinion in Neurobiology. 2004;14:37–44. doi: 10.1016/j.conb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- DeLeo JA, Tawfik VL, LaCroix-Fralish MZ. The tetrapartite synapse: Path to CNS sensitization and chronic pain. Pain. 2006;122:17–21. doi: 10.1016/j.pain.2006.02.034. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–1135. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Research. 1991;565:1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Kreutzberg GW. Delayed astrocyte reaction following facial nerve axotomy. Journal of Neurocytology. 1988;17:209–220. doi: 10.1007/BF01674208. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Tetzlaff W, Streit WJ, Kreutzberg GW. Microglial cells but not astrocytes undergo mitosis following rat facial nerve axotomy. Neuroscience Letters. 1988;85:317–321. doi: 10.1016/0304-3940(88)90585-x. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. Journal of Neuroscience. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall LL, Borke RC, Anders JJ. Transection or electrical stimulation of the hypoglossal nerve increases glial fibrillary acidic protein immunoreactivity in the hypoglossal nucleus. Brain Research. 1989;490:157–161. doi: 10.1016/0006-8993(89)90443-5. [DOI] [PubMed] [Google Scholar]
- Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. Journal of Neuroimmunology. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. Journal of Neuroscience. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Ransom BR. Neuroglia. 2nd Edition. Oxford University Press; 2005. [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends in Neurosciences. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Differential effects of glutamate receptor antagonists on dorsal horn neurons responding to colorectal distension in a neonatal colon irritation rat model. World Journal of Gastroenterology. 2005;11:6495–6502. doi: 10.3748/wjg.v11.i41.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood LL, Silbert LH, Laudenslager ML, Watkins LR, Maier SF. Anesthesia-induced modulation of in vivo antibody levels: a study of pentobarbital, chloral hydrate, methoxyflurane, halothane, and ketamine/xylazine. Anesthesia and Analgesia. 1993;77:769–774. doi: 10.1213/00000539-199310000-00020. [DOI] [PubMed] [Google Scholar]
- Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, et al. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. European Journal of Neuroscience. 2005;22:2775–2782. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, et al. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Research. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. European Journal of Neuroscience. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- Owolabi SA, Saab CY. Fractalkine and minocycline alter neuronal activity in the spinal cord dorsal horn. FEBS Letters. 2006;580:4306–4310. doi: 10.1016/j.febslet.2006.06.087. [DOI] [PubMed] [Google Scholar]
- Polazzi E, Contestabile A. Reciprocal interactions between microglia and neurons: from survival to neuropathology. Reviews in the Neurosciences. 2002;13:221–242. doi: 10.1515/revneuro.2002.13.3.221. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. Journal of Pharmacology and Experimental Therapeutics. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. European Journal of Neuroscience. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Saab CY, Park YC, Al-Chaer ED. Thalamic modulation of visceral nociceptive processing in adult rats with neonatal colon irritation. Brain Research. 2004;1008:186–192. doi: 10.1016/j.brainres.2004.01.083. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Nervenkitt: notes on the history of the concept of neuroglia. Glia. 1988;1:2–9. doi: 10.1002/glia.440010103. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Sun YN, Luo JY, Rao ZR, Lan L, Duan L. GFAP and Fos immunoreactivity in lumbo-sacral spinal cord and medulla oblongata after chronic colonic inflammation in rats. World Journal of Gastroenterology. 2005;11:4827–4832. doi: 10.3748/wjg.v11.i31.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proceedings of the National Academy of Sciences of the U.S.A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Graeber MB, Bisby MA, Kreutzberg GW. Increased glial fibrillary acidic protein synthesis in astrocytes during retrograde reaction of the rat facial nucleus. Glia. 1988;1:90–95. doi: 10.1002/glia.440010110. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. European Journal of Neuroscience. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Vilhardt F. Microglia: phagocyte and glia cell. International Journal of Biochemistry and Cell Biology. 2005;37:17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiological Reviews. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends in Neurosciences. 2001a;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Spinal cord glia: new players in pain. Pain. 2001b;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proceedings of the National Academy of Sciences of the U.S.A. 1999;96:7675–7679. doi: 10.1073/pnas.96.14.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]