Abstract
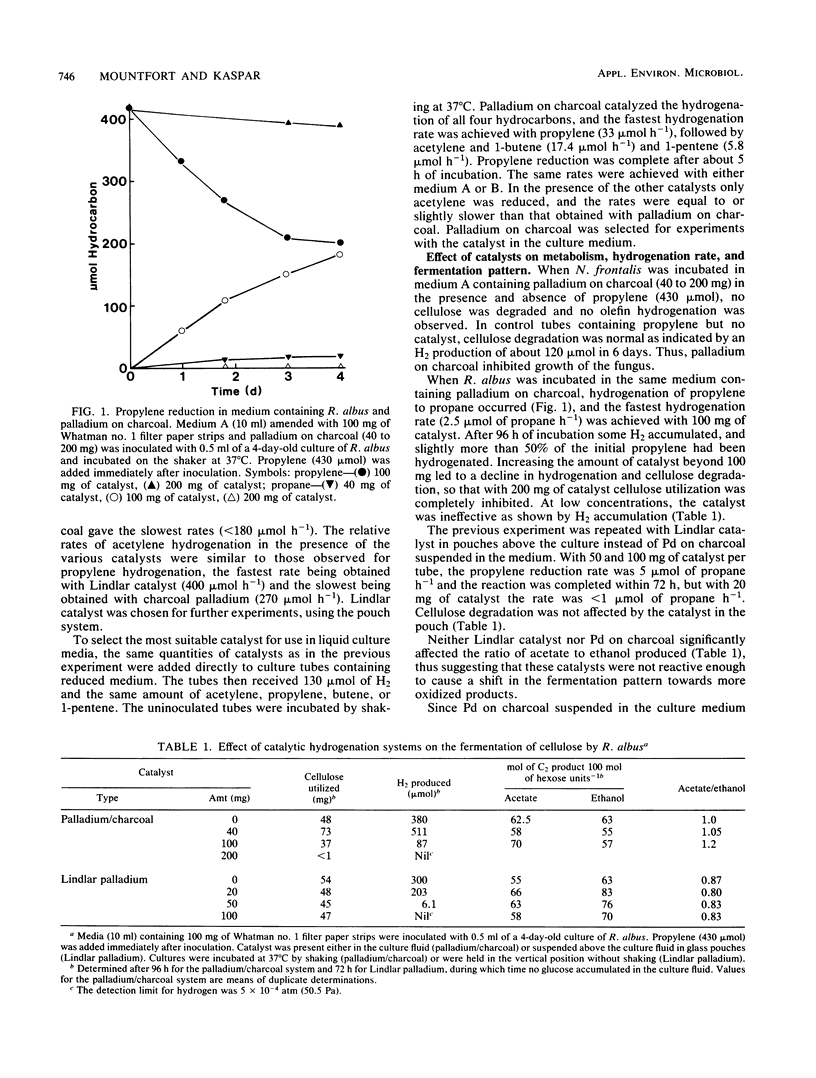
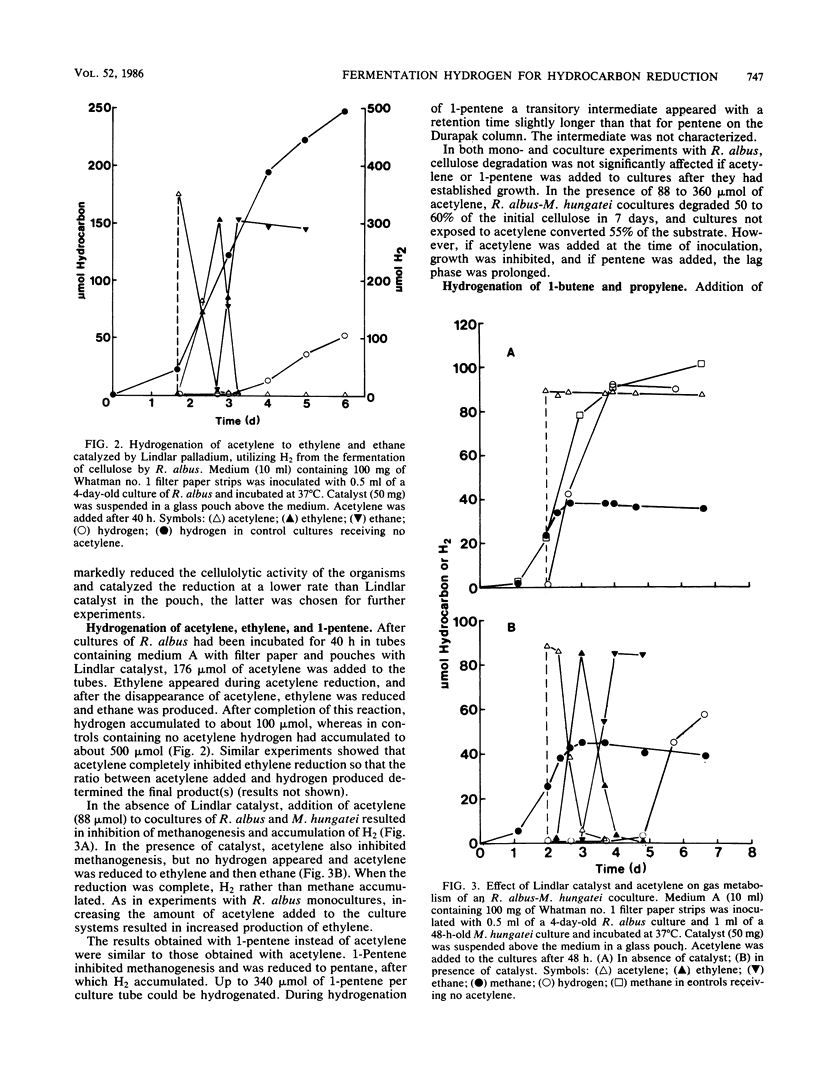
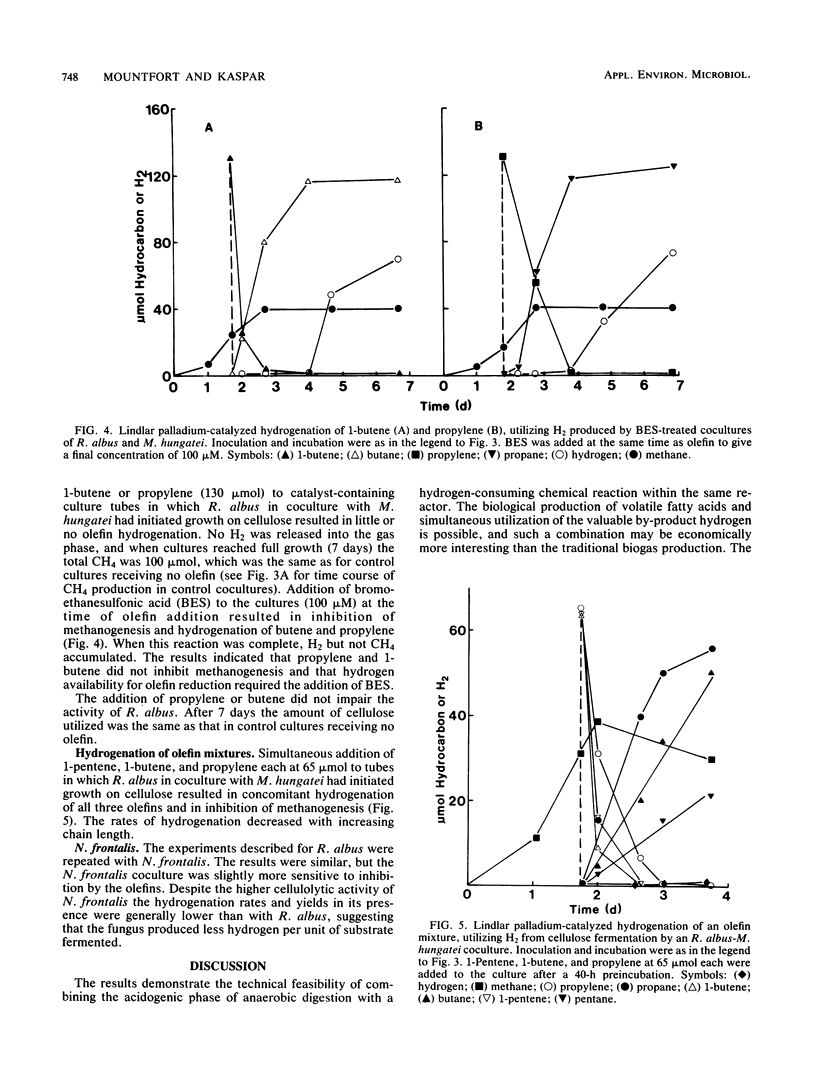
Among five hydrogenation catalysts, palladium on charcoal was the most reactive one when suspended in anaerobic culture medium, and Lindlar catalyst (Pd on CaCO3) was the most reactive one when suspended in the gas phase of culture tubes. Palladium on charcoal in the culture medium (40 to 200 mg 10 ml−1) completely inhibited growth of Neocallimastix frontalis and partly inhibited Ruminococcus albus. Lindlar catalyst (40 to 200 mg per tube) suspended in a glass pouch above the culture medium did not affect the rate of cellulose degradation or the ratio of fermentation products by these organisms. Acetylene added to tubes containing Lindlar catalyst in pouches, and either of the two organisms in monoculture or coculture with Methanospirillum hungatei, was reduced to ethylene and then ethane, followed by hydrogen production. Similar results were obtained with 1-pentene. Neither acetylene nor 1-pentene affected cellulose degradation but both inhibited methanogenesis. In the presence of Lindlar catalyst and propylene or 1-butene, fermenter-methanogen cocultures continued to produce methane at the same rate as controls and no olefin reduction occurred. Upon addition of bromoethanesulfonic acid, methanogenesis stopped and olefin reduction took place followed by hydrogen evolution. In a gas mixture consisting of propylene, 1-butene, and 1-pentene, the olefins were reduced at rates which decreased with increasing molecular size. These results demonstrate the technical feasibility of combining in one reactor the volatile fatty acid production by anaerobic digestion with chemical catalyst-mediated reductions, using the valuable by-product hydrogen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. R., LeGall L., Peck H. D. Evidence for the periplasmic location of hydrogenase in Desulfovibrio gigas. J Bacteriol. 1974 Nov;120(2):994–997. doi: 10.1128/jb.120.2.994-997.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Conrad R., Phelps T. J., Zeikus J. G. Gas metabolism evidence in support of the juxtaposition of hydrogen-producing and methanogenic bacteria in sewage sludge and lake sediments. Appl Environ Microbiol. 1985 Sep;50(3):595–601. doi: 10.1128/aem.50.3.595-601.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGGETT A. S., NIXON D. A. Use of glucose oxidase, peroxidase, and O-dianisidine in determination of blood and urinary glucose. Lancet. 1957 Aug 24;273(6991):368–370. doi: 10.1016/s0140-6736(57)92595-3. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Wuhrmann K. Kinetic parameters and relative turnovers of some important catabolic reactions in digesting sludge. Appl Environ Microbiol. 1978 Jul;36(1):1–7. doi: 10.1128/aem.36.1.1-7.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. Denitrification, acetylene reduction, and methane metabolism in lake sediment exposed to acetylene. Appl Environ Microbiol. 1979 Sep;38(3):486–493. doi: 10.1128/aem.38.3.486-493.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham M. J., Wolin M. J. Fermentation of cellulose by Ruminococcus flavefaciens in the presence and absence of Methanobacterium ruminantium. Appl Environ Microbiol. 1977 Sep;34(3):297–301. doi: 10.1128/aem.34.3.297-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A., Mays E. L., Tiedje J. M. Carbon and electron flow in mud and sandflat intertidal sediments at delaware inlet, nelson, new zealand. Appl Environ Microbiol. 1980 Apr;39(4):686–694. doi: 10.1128/aem.39.4.686-694.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomei F. A., Maki J. S., Mitchell R. Interactions in syntrophic associations of endospore-forming, butyrate-degrading bacteria and h(2)-consuming bacteria. Appl Environ Microbiol. 1985 Nov;50(5):1244–1250. doi: 10.1128/aem.50.5.1244-1250.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Fermentation of cellulose and cellobiose by Clostridium thermocellum in the absence of Methanobacterium thermoautotrophicum. Appl Environ Microbiol. 1977 Feb;33(2):289–297. doi: 10.1128/aem.33.2.289-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



