Abstract
Fusion genes have been identified as chromosomal rearrangements in certain cancers, such as leukaemia, lymphoma, and sarcoma. The EML4–ALK (EML4: echinoderm microtubule-associated-protein-like 4; ALK: anaplastic lymphoma kinase) fusion gene has been identified as an oncogene in non-small-cell lung cancer (NSCLC). This study examined the presence of this fusion transcript in gastrointestinal and breast cancers. We evaluated the expression of the EML4–ALK transcript in 104 lung cancer cases and in 645 gastrointestinal and breast cancer samples. Only one of the lung cancer samples tested positive for the EML4–ALK fusion transcript, whereas none were detected in 555 gastrointestinal and 90 breast cancer cases. Our data suggest that the EML4–ALK fusion transcript is not present in gastrointestinal or breast cancers and is specific to NSCLC.
Keywords: EML4–ALK , fusion transcript, non-small-cell lung cancer, gastrointestinal cancer, breast cancer
Chromosomal aberrations, in particular, translocations and their corresponding gene fusions, are common occurrences in certain cancers, such as leukaemia, lymphoma, and sarcoma; however, these abnormalities have not been as readily identified in solid cancers (Mitelman et al, 2007; Rikova et al, 2007). Two characteristics of chromosomal rearrangements, gene fusions, and fusion proteins make them ideal targets for cancer diagnosis and treatment. First, chromosomal rearrangements and gene fusions are specific to tumour cells, are associated with tumour development, and are not found in normal cells and tissues. Target cancer cells are easily detected with high specificity using reverse transcription-polymerase chain reaction (RT–PCR), with primers designed for the corresponding gene fusion partners. Second, treatments targeting fusion proteins are tumour-specific and show fewer adverse effects. For example, imatinib mesylate (Gleevec), which targets the Bcr–Abl fusion protein, is a highly effective treatment for chronic myelogenous leukaemia (Druker et al, 1996, 2006). Recently, Soda et al (2007) reported the existence of a fusion gene in non-small-cell lung cancer (NSCLC). They reported that a small inversion within chromosome 2p results in the formation of a fusion comprising portions of the echinoderm microtubule-associated-protein-like 4 (EML4) gene and the anaplastic lymphoma kinase (ALK) gene. This fusion was detected in 5 out of the 75 cases studied. The identification of this and other chromosomal aberrations would be an important step in understanding the mechanisms underlying the development of solid cancers. Therefore, this study was undertaken to evaluate the presence of the EML4–ALK transcript in 749 cases of lung cancer and 2 major solid carcinomas (gastrointestinal and breast cancers). We established that the EML4–ALK gene alteration is not found in both these carcinomas and is specific to NSCLC.
MATERIALS AND METHODS
Clinical samples
Fresh surgical specimens were obtained from 749 patients, as described in Table 1. The patients had undergone surgery at the Kyushu University Hospital at Beppu, Hiroshima Red Cross Hospital, or Kagoshima University Hospital. Written informed consent was obtained from all patients according to the guidelines approved by the Institutional Research Board, and this study was conducted under the supervision of the ethical board of Kyushu University.
Table 1. Specimens examined for EML4–ALK transcript.
| Number | Positive case | |
|---|---|---|
| Lung | 104 | 1 |
| Colorectum | 96 | 0 |
| Stomach | 96 | 0 |
| Oesophagus | 112 | 0 |
| Breast | 90 | 0 |
| Liver | 232 | 0 |
| Cholangiocellular | 11 | 0 |
| Pancreas | 8 | 0 |
| Total | 749 | 1 |
ALK=anaplastic lymphoma kinase; EML4=echinoderm microtubule-associated-protein-like 4.
RNA preparation and reverse transcription
Total RNA was isolated using a modified acid guanidinium-phenol-chloroform procedure with DNase. Complementary DNA was synthesized from 2.5 μg of total RNA, as described previously (Inoue et al, 1995).
RT–PCR of EML4–ALK fusion transcript
The following primers were designed to amplify 247 bp of the EML–ALK fusion transcript (Soda et al, 2007): 5′-TGCAGTGTTTAGCATTCTTGGGG-3′ (forward) and 5′-TCTTGCCAGCAAAGCAGTAGTTGG-3′ (reverse).
Either of the fused gene variants (1 or 2) could be amplified with the primer sets described by Soda et al (2007).
The primers for nested RT–PCR were also employed: 5′-GCAGTGTTTAGCATTCTTGGGGA-3′ (forward) and 5′-CTTGCCAGCAAAGCAGTAGTTGGG-3′ (reverse).
The forward primer was located in exon 2 of the EML gene, whereas the reverse primer was located in exon 3 of ALK. Amplification was performed for 35 cycles of 30 s at 95°C, 30 s at 66°C, and 1 min at 72°C. A 10 μl aliquot of each reaction mixture was size-fractionated in 2% agarose gel and visualised by ethidium bromide staining. Amplification of the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene transcript was used as a positive control (of RNA integrity and assay conditions). GAPDH was amplified using the following cycling parameters: 22 cycles of 30 s at 95°C, 30 s at 56°C, and 30 s at 72°C. The following GAPDH primer sequences were used: 5′-TTGGTATCGTGGAAGGACTCA-3′ (forward) and 5′-TGTCATCATATTTGGCAGGTT-3′ (reverse) (Inoue et al, 1995). The RT–PCR product was cloned into pCR vector using TA Cloning Kit Dual Promoter pCR II (Invitrogen Corp., Carlsbad, CA, USA) and sequenced with ABI PRISM 3100-Avant Genetic Analyzer (Applied Biosystems, Foster, CA, USA).
DNA extraction
Frozen tissue was dissolved in 200 μg of buffer ATL containing 10% proteinaseK, and genomic DNA was extracted and purified using QIAamp DNA Micro Kit (Qiagen, Hilden, Germany), in accordance with the manufacturer’s protocols.
Long PCR of EML4–ALK fusion gene
The following primers were designed to amplify the genomic sequence flanking the breakpoint of the EML4–ALK fusion gene (Soda et al, 2007): 5′-CCACACCTGGGAAAGGACCTAAAG-3′ (forward) and 5′-AGCTTGCTCAGCTTGTACTCAGGG-3′ (reverse). Amplification was performed for 35 cycles of 30 s at 94°C, 30 s at 60°C, and 5 min at 72°C. A 20 μl aliquot of each reaction mixture was size-fractionated in 2% agarose gel and visualised by ethidium bromide staining.
RESULTS AND DISCUSSION
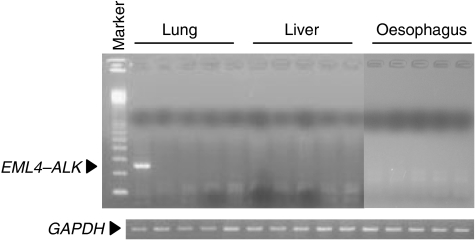
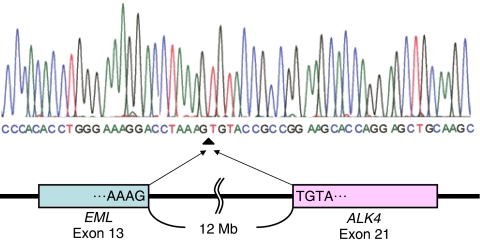
Expression of the EML4–ALK fusion gene was studied in 749 solid tumours (Table 1). A 247 bp transcript was amplified in only 1 out of the 104 lung cancers studied (Figure 1). Sequencing of this transcript confirmed that it was the product of the EML4–ALK fusion (type 1 variant) (Figure 2). However, this transcript was not detected in 555 gastrointestinal and 90 breast cancers. The RT–PCR experiments were repeated three times, including one using nested inner primer set; we thereby confirmed the above results. Thus, the EML4–ALK fusion gene appears to be specific to NSCLC. Very recently, Inamura et al (2008) investigated the presence of EML4–ALK fusion in lung cancers: 149 adenocarcinomas, 48 squamous cell carcinomas, 3 large-cell neuroendocrine carcinomas, and 21 small-cell carcinomas. They reported that 5 out of 149 adenocarcinomas showed EML4–ALK fusion, but this was not found in carcinomas of other types. The case expressing EML4–ALK in our report was also adenocarcinoma. The EML4–ALK fusion gene is thus considered to be specific to NSCLC, especially adenocarcinomas.
Figure 1.
Screening of specimens for EML4–ALK mRNA. Representative cases of lung, liver, and oesophageal carcinoma were subjected to reverse transcription-polymerase chain reaction with primers for EML4–ALK fusion transcript. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA is also shown. Marker, 100 bp DNA ladder.
Figure 2.
Schematic of reverse transcription-polymerase chain reaction revealing fusion of EML4 with ALK in a case with non-small-cell lung cancer (adenocarcinoma). Line graph shows the position and automated DNA sequencing of the fusion points (identical to the variant 1 transcript).
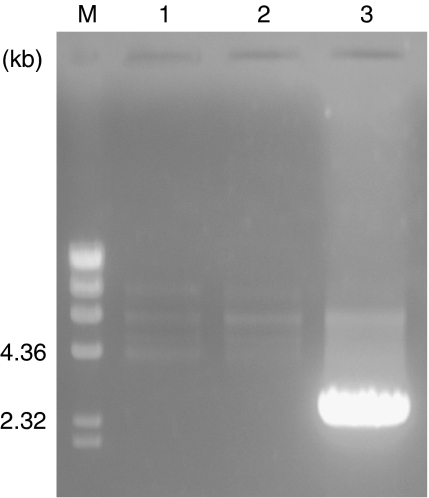
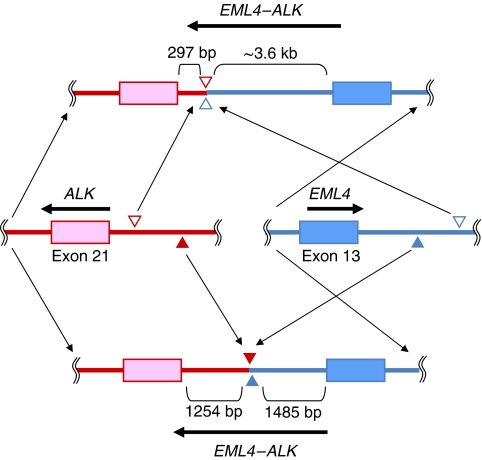
We next surveyed the genomic alterations in nine cases of NSCLC, including one that was positive for EML4–ALK fusion transcript. A 2.5 kb PCR product was detected only in the EML4–ALK-positive case (Figure 3). The size of the long PCR product was different from the one originally reported by Soda, indicating that our case had a different breakpoint. The genomic DNA involved by the alteration was sequenced to identify the breakpoints in the EML4 and ALK genes. As a result, EML4 was disrupted at a position 1485 bp downstream of exon 13 and was ligated to a position 1254 bp upstream of exon 21 of ALK (Figure 4). In the report by Soda, EML4 was disrupted at a position approximately 3.6 bp downstream of exon 13 and was ligated to a position 297 bp upstream of exon 21 of ALK. Thus, we confirmed the difference of genomic rearrangements between the present case and the original report by Soda.
Figure 3.
EML4–ALK genomic alterations in non-small-cell lung cancer. Results from three representative cases are shown. EML4–ALK genomic fusion was found in case 3. Marker, λ/HindIII.
Figure 4.
The breakpoints in EML4 and ALK genes. The upper figure shows the structure of EML4–ALK variant 1 described by Soda et al (2007) and the lower figure shows the structure of EML4–ALK that we found. The middle figure shows a wild-type EML4–ALK. Filled and open arrows indicate breakpoints.
Earlier, fusion genes were identified based on the presence of large-scale chromosomal rearrangements. However, genome project completions and advances in microarray technology have revealed chromosomal rearrangements in several kinds of cancers (Mitelman et al, 2004; Hirasaki et al, 2007). The report of the TMPRSS2–ERG fusion in prostate cancer by Tomlins et al (2005) is probably the first description of such a gene fusion in solid cancers. This altered gene was recognized in 22 out of 24 examined cases, and activity of the TMPRSS2–ERG fusion protein might be correlated with prostate cancer development. Of course, as this gene does not exist in normal cells, it could prove useful for cancer diagnosis and treatment. The TMPRSS2–ERG fusion does not show a chromosomal translocation, but originates from a 2.7 Mb deletion in chromosome 19 (Tomlins et al, 2005, 2007). The EML4–ALK fusion gene reported by Soda et al (2007) is also derived from a genomic inversion of less than 12 Mb in chromosome 2. These alterations (deletions or inversion) are too small to be detected by traditional cytogenetic techniques. Microarrays allow a more detailed analysis and the possibility of detecting novel fusion genes in the absence of chromosomal translocations.
The TMPRSS2–ERG fusion gene is specific to prostate cancer, which may be due to the strong induction of TMPRSS2 by androgen (Perner et al, 2006). In contrast, the reason behind the NSCLC specificity of EML4–ALK is unclear. This alteration was not detected in two of the most prevalent carcinomas, gastrointestinal and breast cancers. However, as mentioned above, there is a possibility that novel fusion genes will be identified, which can open a new era in the diagnosis and treatment of these common solid cancers.
Acknowledgments
We thank T Shimooka, K Ogata, N Kasagi, and Y Nakagawa for their excellent technical assistance. This study was supported by Grant-in-Aid for Scientific Research (S) (17109013), Grant-in-Aid for Scientific Research (B) (18390367), and Grant-in-Aid for Exploratory Research (18659384) from the Japan Society for the Promotion of Science and Core Research for Evolutional Science and Technology of Japan Science and Technology.
References
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA, IRIS Investigators (2006) Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355: 2408–2417 [DOI] [PubMed] [Google Scholar]
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB (1996) Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr–Abl positive cells. Nat Med 2: 561–566 [DOI] [PubMed] [Google Scholar]
- Hirasaki S, Noguchi T, Mimori K, Onuki J, Morita K, Inoue H, Sugihara K, Mori M, Hirano T (2007) BAC clones related to prognosis in patients with esophageal squamous carcinoma: an array comparative genomic hybridization study. Oncologist 12: 406–417 [DOI] [PubMed] [Google Scholar]
- Inamura K, Takeuchi K, Togashi Y, Nomura K, Ninomiya H, Okui M, Satoh Y, Okumura S, Nakagawa K, Soda M, Choi YL, Niki T, Mano H, Ishikawa Y (2008) EML4–ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 3: 13–17 [DOI] [PubMed] [Google Scholar]
- Inoue H, Mori M, Honda M, Li J, Shibuta K, Mimori K, Ueo H, Akiyoshi T (1995) The expression of tumor-rejection antigen ‘MAGE’ genes in human gastric carcinoma. Gastroenterology 109: 1522–1525 [DOI] [PubMed] [Google Scholar]
- Mitelman F, Johansson B, Mertens F (2004) Fusion genes and rearranged genes as a linear function of chromosome aberrations in cancer. Nat Genet 36: 331–334 [DOI] [PubMed] [Google Scholar]
- Mitelman F, Johansson B, Mertens F (2007) The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer 7: 233–245 [DOI] [PubMed] [Google Scholar]
- Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S, Tchinda J, Tomlins SA, Hofer MD, Pienta KG, Kuefer R, Vessella R, Sun XW, Meyerson M, Lee C, Sellers WR, Chinnaiyan AM, Rubin MA (2006) TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res 66: 8337–8341 [DOI] [PubMed] [Google Scholar]
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ (2007) Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131: 1190–1203 [DOI] [PubMed] [Google Scholar]
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H (2007) Identification of the transforming EML4–ALK fusion gene in non-small-cell lung cancer. Nature 448: 561–566 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, Yu J, Wang L, Montie JE, Rubin MA, Pienta KJ, Roulston D, Shah RB, Varambally S, Mehra R, Chinnaiyan AM (2007) Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 448: 595–599 [DOI] [PubMed] [Google Scholar]
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310: 644–648 [DOI] [PubMed] [Google Scholar]