Abstract
We confirmed the strong association of hepatoblastoma with very low birth weight (relative risk <1000 g vs ⩾2000 g=25.6; 95% confidence interval: 7.70–85.0) and demonstrated independent associations with congenital abnormalities and maternal Asian race in a population-based Minnesota study that included 36 cases and 7788 controls.
Keywords: paediatrics, case–control studies, medical record linkage, prematurity
Hepatoblastoma is a rare embryonal tumour that comprises most cases of liver cancer in children aged 0–5 years in the United States (Ries et al, 1999). Although its causes are mostly obscure, it is apparent that very low birth weight (VLBW), generally defined as <1500 g, is a potent risk factor (Tanimura et al, 1998; Reynolds et al, 2004; Ansell et al, 2005; McLaughlin et al, 2006). Hepatoblastoma incidence doubled between 1975 and 1999 (Spector et al, 2004), possibly related to the concomitant rise in prevalence of VLBW infants and a marked drop in their mortality (CDC, 2002). These observations may indicate that some component of treatment for prematurity is carcinogenic or, alternatively, that the aetiology may overlap with that of VLBW. To expand a sparse literature, we examined the relation between hepatoblastoma and birth characteristics in Minnesota.
MATERIALS AND METHODS
The methods used in this case-cohort study have previously been described (Puumala et al, 2008). Briefly, we matched Minnesota Cancer Surveillance System (MCSS) records of incident first cancers diagnosed in children aged 28 days to 14 years during 1988–2004 to birth records using probabilistic record linkage (Jaro, 1995). For each of the 2188 successfully linked cases (out of 2655 total) we randomly selected four birth records of children born in the same year and who survived at least 28 days past birth; this comparison group of 8752 subjects is referred to as the subcohort. In this analysis, matching cases were compared to all subcohort members born in 1982 (i.e., the earliest year a case would have been born) or later to improve study power.
Exposure variables derived from Minnesota birth records are shown in Table 1. Some variables were not available during the entire study period. Birth weight was divided into categories of <1000, 1000–1999, and ⩾2000 g to maximize the number of cases in each stratum. Race was classified as white or non-white. Other variables were categorized using customary cut offs (Ries et al, 1999).
Table 1. Associations of demographic, pregnancy, and birth characteristics with hepatoblastoma adjusted for birth weight, birth year, and sex — Minnesota, 1982–2004.
| Subcohort (n=7788) | Cases (n=36) | |||
|---|---|---|---|---|
| Characteristic | N (%) | N (%) | HRa | 95% CI |
| Birth characteristics | ||||
| Birth weight (g) | ||||
| >2000 | 7606 (98.0) | 28 (77.8) | 1 | |
| 1000–1999 | 124 (1.6) | 4 (11.1) | 9.15 | 3.09–27.1 |
| <1000 | 31 (0.4) | 4 (11.1) | 25.6 | 7.70–85.0 |
| Sex | ||||
| Female | 3778 (48.5) | 11 (30.6) | 1 | |
| Male | 4010 (51.5) | 25 (69.4) | 2.03 | 0.98–4.19 |
| Gestational age (weeks)b | ||||
| ⩾37 | 6888 (91.6) | 25 (69.4) | 1 | |
| <37 | 631 (8.4) | 11 (30.6) | 1.70 | 0.56–5.13 |
| Size for gestational age | ||||
| Small | 252 (3.4) | 0 (0.0) | — | |
| Average | 5427 (72.4) | 29 (80.6) | — | |
| Large | 1821 (24.3) | 7 (19.4) | — | |
| Multiple birth | ||||
| No | 7592 (97.5) | 34 (94.4) | — | |
| Yes | 196 (2.5) | 2 (5.6) | — | |
| Apgar score (1 min) | ||||
| >7 | 5725 (76.6) | 19 (52.8) | 1 | |
| ⩽7 | 1746 (23.4) | 17 (47.2) | 1.99 | 0.95–4.16 |
| Apgar score (5 min) | ||||
| >7 | 7192 (96.4) | 29 (80.6) | 1 | |
| <7 | 265 (3.6) | 7 (19.4) | 2.24 | 0.70–7.11 |
| Assisted ventilationc | ||||
| No | 4549 (98.3) | 25 (86.2) | 1 | |
| Yes | 79 (1.7) | 4 (13.8) | 2.00 | 0.49–8.19 |
| Congenital abnormality | ||||
| No | 7676 (98.6) | 31 (86.1) | 1 | |
| Yes | 112 (1.4) | 5 (13.9) | 5.87 | 1.88–18.3 |
| Index pregnancy history | ||||
| Adequacy of prenatal care | ||||
| Adequate | 4530 (67.9) | 24 (75.0) | 1 | |
| Intermediate | 1488 (22.3) | 4 (12.5) | 0.61 | 0.21–1.78 |
| Inadequate | 658 (9.9) | 4 (12.5) | 1.11 | 0.36–3.43 |
| Weight gain (pounds)c | ||||
| ⩽24 | 1029 (26.7) | 7 (25.9) | 1 | |
| 25–30 | 1295 (33.6) | 10 (37.0) | 1.36 | 0.48–3.83 |
| >30 | 1526 (39.6) | 10 (37.0) | 1.40 | 0.48–4.07 |
| Intrauterine procedures | ||||
| No | 7271 (97.4) | 32 (88.9) | 1 | |
| Yes | 193 (2.6) | 4 (11.1) | 2.39 | 0.68–8.41 |
| Induction of laborc | ||||
| No | 3439 (71.6) | 20 (66.7) | 1 | |
| Yes | 1361 (28.4) | 10 (33.3) | 1.32 | 0.60–2.93 |
| Type of delivery | ||||
| Vaginal | 6162 (82.0) | 26 (72.2) | 1 | |
| Caesarean section | 1355 (18.0) | 10 (27.8) | 1.26 | 0.58–2.77 |
| Maternal reproductive history | ||||
| Prior live births | ||||
| 0 | 3062 (39.9) | 16 (44.4) | 1 | |
| 1–2 | 3799 (49.5) | 11 (30.6) | 0.62 | 0.28–1.35 |
| ⩾3 | 808 (10.5) | 9 (25.0) | 2.07 | 0.87–4.92 |
| Interval since last live birth (years) | ||||
| No prior birth | 3062 (40.1) | 16 (44.4) | — | |
| ⩽3 | 2966 (38.9) | 18 (50.0) | — | |
| >3 | 1600 (21.0) | 2 (5.6) | — | |
| Prior fetal losses | ||||
| None | 5919 (77.5) | 30 (83.3) | 1 | |
| Any | 1717 (22.5) | 6 (16.7) | 0.60 | 0.24–1.49 |
| Parental demographics | ||||
| Mother's age (years) | ||||
| <25 | 2358 (30.3) | 11 (30.6) | 1.27 | 0.53–3.08 |
| 25–29 | 2708 (34.8) | 10 (27.8) | 1 | |
| ⩾30 | 2722 (35.0) | 15 (41.7) | 1.31 | 0.57–3.01 |
| Father's age (years) | ||||
| <30 | 3347 (47.5) | 11 (33.3) | 1 | |
| ⩾30 | 3693 (52.5) | 22 (66.7) | 1.55 | 0.74–3.25 |
| Mother's race | ||||
| White | 7010 (90.9) | 30 (83.3) | 1 | |
| Non-white | 700 (9.1) | 6 (16.7) | 1.56 | 0.63–3.89 |
| Mother's ethnicityc | ||||
| Non-Hispanic | 4475 (96.5) | 26 (86.7) | 1 | |
| Hispanic | 160 (3.5) | 4 (13.3) | 2.96 | 0.88–10.0 |
| Mother's birthplace | ||||
| United States | 7297 (93.8) | 28 (77.8) | 1 | |
| Elsewhere | 485 (6.2) | 8 (22.2) | 3.55 | 1.51–8.32 |
| Father's race | ||||
| White | 6381 (92.5) | 28 (87.5) | 1 | |
| Nonwhite | 521 (7.5) | 4 (12.5) | 1.44 | 0.49–4.29 |
| Father's ethnicityc | ||||
| Non-Hispanic | 3996 (97.0) | 24 (85.7) | 1 | |
| Hispanic | 125 (3.0) | 4 (14.3) | 4.18 | 1.22–14.3 |
| Mother's education (years) | ||||
| <12 | 782 (10.6) | 8 (22.9) | 1.60 | 0.64–4.02 |
| 12 | 2706 (36.8) | 14 (40.0) | 1 | |
| >12 | 3867 (52.6) | 13 (37.1) | 0.61 | 0.28–1.32 |
| Father's education (years) | ||||
| ⩽12 | 2840 (44.2) | 13 (43.3) | 1 | |
| >12 | 3587 (55.8) | 17 (56.7) | 1.03 | 0.49–2.16 |
Abbreviations: CI=confidence interval; HR= Hazard ratios; LNMP=last normal menstrual period
Hazard ratios adjusted for birth weight, birth year, and sex.
Imputed gestational age based on LNMP when available and physician's estimate when LNMP was missing.
Collected in birth years 1989–2004.
We calculated hazard ratios (HRs) and 95% confidence intervals (CIs) using stratified Cox regression models using SAS 9.1 (SAS institute Inc., Cary, NC, USA) (Langholz and Jiao, 2007). The number of cases precluded a full multivariate model. However, birth weight is a known risk factor; all variables were modelled adjusting for birth weight as well as for year of birth and sex.
RESULTS
Of 39 cases of hepatoblastoma identified by MCSS, 36 (92.3%) linked to birth records. Cases were compared to 7788 members of the subcohort. Exposure frequencies and adjusted HRs are presented in Table 1; where cell size was <4, we reported frequencies but did not calculate HRs. There were strong associations with birth weight <1000 g (HR=25.6; 95% CI: 7.70–85.0) and 1000–1999 g (HR=9.15; 95% CI: 3.09–27.1) compared to ⩾2000 g, their magnitude with each category of low birth weight was lessened, but remained highly significant, when adjusting for covariates (data not shown). Significant univariate associations were noted with maternal and paternal Hispanic ethnicity, maternal birthplace outside the US, intrauterine procedures during pregnancy, male sex, gestational age <37 weeks, 1 and 5 min apgar scores, assisted ventilation, and congenital abnormalities (data not shown). However, only associations with congenital abnormalities (HR=5.87; 95% CI: 1.88–18.3), paternal Hispanic ethnicity (HR=4.18; 95% CI: 1.22–14.3), and maternal birthplace outside the US (HR=3.55; 95% CI: 1.51–8.32) remained significant after adjustment. The association of foreign maternal birthplace reflected the disproportionate number of cases with mothers from Southeast Asia. Accordingly, there was a significant adjusted association of maternal Asian race with hepatoblastoma (HR=3.86; 95% CI: 1.30–11.52).
DISCUSSION
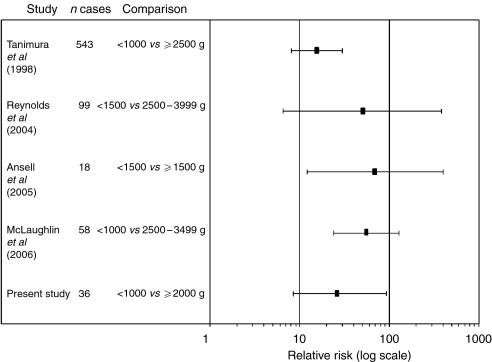
Very low birth weight has emerged over the past 15 years as a potent risk factor for hepatoblastoma (Feusner et al, 1998; Tanimura et al, 1998; Reynolds et al, 2004; Ansell et al, 2005; McLaughlin et al, 2006). We have now confirmed this association in the paediatric population of Minnesota. Unadjusted relative risks between 16 and 70 comparing very low to moderate birth weight children have been reported in studies from the United Kingdom, Japan, and now three US states (Figure 1). This striking association thus appears across industrialized nations.
Figure 1.
Relative risk of hepatoblastoma comparing very low to moderate birth weight children in five studies.
Rather than being causative per se, VLBW likely signals the involvement of correlated factors. Limited multivariate analyses in this study and others (Reynolds et al, 2004; McLaughlin et al, 2006) have begun to tease apart the role of other characteristics from that of VLBW. Notably, preterm birth (<37 weeks) was not an independent risk factor in any of the studies after adjustment.
We found a strong association with congenital abnormalities, which was attenuated but still present when controlling for VLBW. One specific abnormality, an omphalocele, was recorded, which may be indicative of Beckwith–Wiedemann syndrome, an overgrowth disorder which is known to increase the risk of hepatoblastoma (Ranzini et al, 1997; DeBaun and Tucker, 1998). The remaining five abnormalities were nonspecific (one central nervous system, one urogenital, and three ‘others’). Higher than expected proportions of congenital abnormalities have previously been noted among cases (Narod et al, 1997; Ansell et al, 2005), but without factoring in birth weight.
The association of maternal Asian race, specifically of Southeast Asian ancestry, with hepatoblastoma was unexpected. Similar findings were not noted in studies in California (Reynolds et al, 2004) and New York (McLaughlin et al, 2006), and there was not an elevated rate in Ho Chi Minh City, Vietnam (Nguyen et al, 2000). Our observation was therefore novel and independent of birth weight. We also observed an independent association of paternal, but not maternal, Hispanic ethnicity. This finding may have been an artifact, as its significance was dependent on two cases for which paternal ethnicity was missing.
Maternal hypertension (Roberts et al, 2003), maternal tobacco use (Horta et al, 1997), and conception by assisted reproductive technology (Schieve et al, 2002) are known to reduce birth weight and have been examined in other studies. An excess of maternal pre-eclampsia, without adjustment for VLBW, has been noted (Ansell et al, 2005). Three previous studies have found that maternal smoking significantly raised offspring risk (Pang et al, 2003; Sorahan and Lancashire, 2004; McLaughlin et al, 2006), whereas a fourth did not (Buckley et al, 1989); associations remained after adjustment for VLBW in two studies (Pang et al, 2003; Spector and Ross, 2003; McLaughlin et al, 2006). Lastly, a ninefold increased risk of hepatoblastoma was reported among children born following infertility treatment, which was independent of birth weight (McLaughlin et al, 2006). That maternal smoking and conception by assisted reproductive technology remain as risk factors after adjustment for VLBW suggests that if causal relationships exist, they operate independently of birth weight.
Use of population-based registry data was the major strength of this study, as any misclassification would most likely be non-differential and HRs would be underestimated. Birth characteristics and delivery methods are reliably recorded in birth records whereas other factors, including congenital abnormalities, are substantially underreported (Northam and Knapp, 2006), contributing to sparse data for several variables. Although cases that occurred among outmigrating children or those who resided in Minnesota during 1982–1987 would have been missed, this occurrence is unlikely given the very low incidence of hepatoblastoma. Lastly, the small number of cases resulted in a reduced ability to perform multivariate analyses.
The strong association of hepatoblastoma with VLBW has been robust to adjustment for other factors. That control for several prenatal determinants of small infant size has not explained the VLBW association may indicate that postnatal treatment is the causative correlate. Although three very small case–control studies of neonatal medical history among VLBW infants preliminarily suggest greater oxygen exposure in cases (Maruyama et al, 1999, 2000; Oue et al, 2003), larger studies are plainly required. Therefore, a multicenter case–control study has been initiated (National Institutes of Health Grant R01CA111355; L Spector, Principal Investigator) that will examine risk factors for hepatoblastoma, with a special focus on VLBW infants.
Acknowledgments
This research was supported by a grant from the Children's Cancer Research Fund, Minneapolis, MN, USA.
References
- Ansell P, Mitchell CD, Roman E, Simpson J, Birch JM, Eden TO (2005) Relationships between perinatal and maternal characteristics and hepatoblastoma: a report from the UKCCS. Eur J Cancer 41: 741–748 [DOI] [PubMed] [Google Scholar]
- Buckley JD, Sather H, Ruccione K, Rogers PC, Haas JE, Henderson BE, Hammond GD (1989) A case–control study of risk factors for hepatoblastoma. A report from the Childrens Cancer Study Group. Cancer 64: 1169–1176 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2002) Infant mortality and low birth weight among black and white infants – United States, 1980–2000. MMWR Morb Mortal Wkly Rep 51: 589–592 [PubMed] [Google Scholar]
- DeBaun MR, Tucker MA (1998) Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr 132: 398–400 [DOI] [PubMed] [Google Scholar]
- Feusner J, Buckley J, Robison L, Ross J, Van Tornout J (1998) Prematurity and hepatoblastoma: more than just an association? J Pediatr 133: 585–586 [DOI] [PubMed] [Google Scholar]
- Horta BL, Victora CG, Menezes AM, Halpern R, Barros FC (1997) Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatr Perinat Epidemiol 11: 140–151 [DOI] [PubMed] [Google Scholar]
- Jaro MA (1995) Probabilistic linkage of large public health data files. Stat Med 14: 491–498 [DOI] [PubMed] [Google Scholar]
- Langholz B, Jiao J (2007) Computational methods for case-cohort studies. Comput Statist Data Anal 51: 3737–3748 [Google Scholar]
- Maruyama K, Ikeda H, Koizumi T, Tsuchida Y (1999) Prenatal and postnatal histories of very low birthweight infants who developed hepatoblastoma. Pediatr Int 41: 82–89 [DOI] [PubMed] [Google Scholar]
- Maruyama K, Ikeda H, Koizumi T, Tsuchida Y, Tanimura M, Nishida H, Takahashi N, Fujimura M, Tokunaga Y (2000) Case–control study of perinatal factors and hepatoblastoma in children with an extremely low birthweight. Pediatr Int 42: 492–498 [DOI] [PubMed] [Google Scholar]
- McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS (2006) Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol 163: 818–828 [DOI] [PubMed] [Google Scholar]
- Narod SA, Hawkins MM, Robertson CM, Stiller CA (1997) Congenital anomalies and childhood cancer in Great Britain. Am J Hum Genet 60: 474–485 [PMC free article] [PubMed] [Google Scholar]
- Nguyen MQ, Nguyen CH, Kramarova E, Parkin DM (2000) Incidence of childhood cancer in Ho Chi Minh City, Vietnam, 1995–1997. Paediatr Perinat Epidemiol 14: 240–247 [DOI] [PubMed] [Google Scholar]
- Northam S, Knapp TR (2006) The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs 35: 3–12 [DOI] [PubMed] [Google Scholar]
- Oue T, Kubota A, Okuyama H, Kawahara H, Nara K, Kawa K, Kitajima H (2003) Hepatoblastoma in children of extremely low birth weight: a report from a single perinatal center. J Pediatr Surg 38: 134–137 [DOI] [PubMed] [Google Scholar]
- Pang D, McNally R, Birch JM (2003) Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. Br J Cancer 88: 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puumala SE, Soler JT, Johnson KJ, Spector LG (2008) Birth characteristics and Wilms tumor in Minnesota. Int J Cancer 122: 1368–1373 [DOI] [PubMed] [Google Scholar]
- Ranzini AC, Day-Salvatore D, Turner T, Smulian JC, Vintzileos AM (1997) Intrauterine growth and ultrasound findings in fetuses with Beckwith-Wiedemann syndrome. Obstet Gynecol 89: 538–542 [DOI] [PubMed] [Google Scholar]
- Reynolds P, Urayama KY, Von Behren J, Feusner J (2004) Birth characteristics and hepatoblastoma risk in young children. Cancer 100: 1070–1076 [DOI] [PubMed] [Google Scholar]
- Ries LA, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR (1999) Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program: Bethesda, MD, Report no. 99–4649 [Google Scholar]
- Roberts JM, Pearson G, Cutler J, Lindheimer M (2003) Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 41: 437–445 [DOI] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS (2002) Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med 346: 731–737 [DOI] [PubMed] [Google Scholar]
- Sorahan T, Lancashire RJ (2004) Parental cigarette smoking and childhood risks of hepatoblastoma: OSCC data. Br J Cancer 90: 1016–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector LG, Feusner JH, Ross JA (2004) Hepatoblastoma and low birth weight. Pediatr Blood Cancer 43: 706. [DOI] [PubMed] [Google Scholar]
- Spector LG, Ross JA (2003) Smoking and hepatoblastoma: confounding by birth weight? Br J Cancer 89: 602; author reply 603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura M, Matsui I, Abe J, Ikeda H, Kobayashi N, Ohira M, Yokoyama M, Kaneko M (1998) Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res 58: 3032–3035 [PubMed] [Google Scholar]