Abstract
Imatinib has revolutionised the treatment of chronic myeloid leukaemia (CML) and gastrointestinal stromal tumours (GIST). Using a nonlinear mixed effects population model, individual estimates of pharmacokinetic parameters were derived and used to estimate imatinib exposure (area under the curve, AUC) in 58 patients. Plasma-free concentration was deduced from a model incorporating plasma levels of alpha1-acid glycoprotein. Associations between AUC (or clearance) and response or incidence of side effects were explored by logistic regression analysis. Influence of KIT genotype was also assessed in GIST patients. Both total (in GIST) and free drug exposure (in CML and GIST) correlated with the occurrence and number of side effects (e.g. odds ratio 2.7±0.6 for a two-fold free AUC increase in GIST; P<0.001). Higher free AUC also predicted a higher probability of therapeutic response in GIST (odds ratio 2.6±1.1; P=0.026) when taking into account tumour KIT genotype (strongest association in patients harbouring exon 9 mutation or wild-type KIT, known to decrease tumour sensitivity towards imatinib). In CML, no straightforward concentration–response relationships were obtained. Our findings represent additional arguments to further evaluate the usefulness of individualising imatinib prescription based on a therapeutic drug monitoring programme, possibly associated with target genotype profiling of patients.
Keywords: imatinib, pharmacokinetics and pharmacodynamics, pharmacogenetics, leukaemia, sarcoma, signal transduction inhibitors
Imatinib mesylate (Glivec®; Novartis, Basel, Switzerland) has revolutionised the treatment and prognosis of chronic myeloid leukaemia (CML) (Druker, 2003; Tothova et al, 2005) and gastrointestinal stromal tumours (GIST) (Steinert et al, 2005). Imatinib was rationally designed to inhibit the PDGF receptor and the BCR-ABL tyrosine kinase (the hallmark of CML), and it was also found to potently inhibit autophosphorylation of the tyrosine kinase receptor c-KIT (involved in the pathogenesis of GIST) (Demetri et al, 2002).
BCR-ABL kinase results from a reciprocal t(9,22) translocation that gives rise to the Philadelphia chromosome in CML (Capdeville et al, 2002). Constitutive activation of c-KIT, associated with various mutation profiles, is observed in the majority of GISTs. The most common mutation site of KIT is located on exon 11. Exon 9 mutation occurs in 10–15% of patients, defining a distinct subset of GISTs having an aggressive clinical behaviour. A few GISTs are characterised by another mutations profile, and about 10% of patients have undetectable mutations (wild type, wt) (Antonescu et al, 2005).
Treatment with tyrosine kinase inhibitors such as imatinib is considered at present to be taken indefinitely, owing to the apparent insensitivity of stem cells to imatinib (Michor et al, 2005; Michor, 2007). Moreover, they are not devoid of inconvenience and toxicity, and resistance occurs in a significant number of patients (Weisberg and Griffin, 2003; Hochhaus and La Rosee, 2004). Finally, such therapies remain fairly expensive at this time (Simonsson et al, 2007). Various adverse events have been described for imatinib, including fluid retention, nausea, skin rash and muscle cramps, with an incidence of more than 50% (grades 1–4) (Cohen et al, 2005; Zalcberg et al, 2005). Cardiotoxicity has also been recently reported (Kerkela et al, 2006). Cellular mechanisms of resistance in CML include point mutations in BCR-ABL gene (up to 40 identified), BCR-ABL amplification or activation of alternative survival signalling pathways (Sawyers et al, 2002; Weisberg and Griffin, 2003). For GISTs, the tumour genotype is a predictor of response to imatinib. Patients harbouring tumours characterised by an exon 11 KIT mutation may benefit from a better response to imatinib compared to other subgroups, notably exon 9 mutants or wt KIT tumours (Heinrich et al, 2003; Debiec-Rychter et al, 2006). Molecular analysis of GISTs thus appears to be an important clinical tool to identify patients at high risk of disease progression. Moreover, about half of the imatinib-resistant GIST patients had acquired secondary mutations in the kinase domain of c-KIT (Antonescu et al, 2005).
Additionally, resistance could also be directly or indirectly caused by an increase in cellular efflux of imatinib, mediated mainly by the drug transporter P-gp (P-glycoprotein) (Mahon et al, 2003; Widmer et al, 2007), or by a decrease in cellular influx, mediated by the uptake carrier hOCT1 (organic cation transporter) (Thomas et al, 2004; Crossman et al, 2005; Wang et al, 2008). Host-dependent mechanisms of resistance have also been incriminated, including modulation of imatinib binding to α1-acid glycoprotein (AGP) in plasma (Gambacorti-Passerini et al, 2000; Gambacorti-Passerini et al, 2003; Larghero et al, 2003) and/or possibly enhanced drug metabolism (Rochat et al, 2008). Finally, nonadherence to imatinib dosage regimen may also play a role in resistance (Tsang et al, 2006). A given dose therefore yields very different circulating concentrations between patients (Widmer et al, 2006; Larson et al, 2008), possibly favouring the selection of resistant cellular clones in case of subtherapeutic drug exposure.
Several pharmacokinetic (PK) studies have been carried out for imatinib. Some have been able to verify the influence of factors such as weight, albuminaemia, haemoglobinaemia or ABCB1 (MDR1) polymorphism on its PK (Judson et al, 2005; Schmidli et al, 2005; Gurney et al, 2007) but not of those such as hepatic enzymes or impaired liver or kidney function (Widmer et al, 2006; Gibbons et al, 2008; Ramanathan et al, 2008). Furthermore, recent evidence suggests that steady-state trough imatinib plasma concentration (TPC) at initiation of therapy is a significant predictor of complete cytogenetic and major molecular responses (Larson et al, 2008). TPC also appears to correlate with response in CML (Picard et al, 2007) as well as in GIST (Demetri et al, 2008). Interestingly, recent studies have begun to investigate the free drug exposure of imatinib (Delbaldo et al, 2006; Widmer et al, 2006). The study from Delbado also explored the relationship between drug exposure (area under the curve, AUC) and effect. It showed that unbound drug exposure was correlated to the haematological toxicity (absolute neutrophil count), but it did not find significant association with treatment efficacy in GIST patients. However, the modulating influence of tumour genetics on the concentration–effect relationship of imatinib, and similar new targeted anticancer drugs, certainly deserves additional evaluation.
The aims of this clinical investigation were as follows: (1) to explore further PK–PD relationships in a population of CML and GIST patients, and (2) to evaluate the specific influence of the target genotype on this relationship in the GIST sub-population.
MATERIALS AND METHODS
Study population and genetic characterisation
The present PK–PD (pharmacokinetic–pharmacodynamic) analysis was performed using data from 58 patients, out of 59 who provided plasma samples collected over 3 years (Widmer et al, 2006). For the present analysis, 280 plasma samples were considered (corresponding to routine visits only). This observational study was approved by the Ethics Committee of the Lausanne Faculty of Medicine. Informed written consent was obtained from all the participants.
The population PK analysis of these data has been published elsewhere (Widmer et al, 2006). The patients included in the present analysis were 38 with GIST and 20 with CML, who received imatinib at various dosage regimens (150–800 mg daily). Peripheral blood samples, obtained under steady-state conditions, were drawn periodically at 1- to 6-month intervals on follow-up visits, along with routine laboratory tests. In addition to accurate dosing and sampling time information, a comprehensive set of demographic and biological data were recorded for each patient, including plasma AGP (Widmer et al, 2006).
Imatinib concentration was measured using a validated method by high-performance liquid chromatography after solid phase extraction (Widmer et al, 2004). The lower limit of quantification is 50 μg l−1, the mean interday coefficient of variation is lower than 2.4% and the range of interday deviations is within −0.6 to +0.7%.
The tumour genetic profile of 20 GIST patients was assessed at the time of the multicentric EORTC Soft Tissue and Bone Sarcoma trial (Debiec-Rychter et al, 2006). Genomic DNA was extracted from sections of paraffin-embedded tumour blocks. Exons 9, 11, 13 and 17 of the KIT gene were amplified by PCR, and the amplicons were analysed for mutations by a combination of DHPLC pre-screening (WAVE DHPLC system, Transgenomic, Cramlington, UK) and bidirectional sequencing (Debiec-Rychter et al, 2004). Specimens that had no detectable KIT mutation (wt KIT) were further tested for PDGFRA exons 12 and 18 mutations. The genetic profiles were coded on a binary scale, with 1=presence of mutation known to confer resistance to imatinib treatment (mutation on KIT exon 9 or wt profile) and 0=absence of such mutation (KIT exon 11 mutation).
Assessment of imatinib exposure
On the basis of model purposely developed at the time of our population PK study (nonlinear mixed effects model; NONMEM) (Widmer et al, 2006), individual post hoc Bayesian estimates of PK parameters were derived for all samples. They were used to calculate maximum likelihood individual drug exposure levels, expressed as AUC (defined as Dose/CL·τ, where CL is the clearance and τ the dosing interval).
Moreover, free parameters (i.e. corresponding to the unbound drug) were estimated using the PK model incorporating plasma AGP levels that we formerly developed (Widmer et al, 2006).
Assessment of clinical response
The therapeutic response was determined at the time of routine follow-up visits. For CML, it was coded on a 3-point scale (complete, CHR=2, partial, PHR=1 and absent, NHR=0 haematological remission, based on white-blood cell count), and was in accordance with RECIST criteria for GIST (Therasse et al, 2000). This criterion was recoded at the time of the efficacy analysis into a 2-point scale (overall responses (OR=1), comprising complete response (CR) plus partial response (PR) vs stable disease (SD) plus progressive disease=0).
As standardised evaluation of typical side effects was not systematically available in our patient's population (e.g. National Cancer Institute's Common Toxicity Criteria, NCI-CTC), the number of side effects experienced by patients was considered instead as a surrogate outcome for toxicity (summarised in a 4-point scale; 0, 1, 2 and 3 or more side effects).
For each blood sample collected, the efficacy and toxicity scores, as well as the Dose considered, were the ones corresponding or reported at the time of sampling. Every score was double-checked before PK–PD analysis.
Statistics
A concentration–effect exploration was first carried out in CML and GIST patients. Associations between log-transformed Dose, as well as total and free AUC or CL, and therapeutic response or toxicity, were explored by ordered logistic regression analysis (Stata® version 8.2, Stata Co., College Station, TX, USA) (Stata Corp, 2003). Although this per-sample analysis allowed taking into account the variations along the time of dose, AGP levels, body weight and age, a more stringent per-patient analysis was also performed to keep away from intrapatient correlation issues. To that purpose, all different data were collapsed in one value for each patient (i.e. average Dose, AUC and CL vs median efficacy and toxicity scores).
In the GIST sub-population, the influence of target mutation profile on the therapeutic response was additionally assessed by incorporating the patients' KIT genotype (coded on the binary scale described above) into the logistic regression model.
The results of the statistical analysis were considered significant at P<0.05, whereas P⩽0.1 values were regarded as indicative of possible trends. As no Bonferroni-like adjustment for multiple testing was applied during this exploratory analysis, P-value nearing 0.05 has, however, to be considered cautiously. Proportional odds ratios related to free drug exposure were derived from the coefficients of the ordered logistic regression model (Stata Corp, 2003). The log2 of PK parameters (AUC and CL) and Dose was used for this calculation to obtain odds ratios corresponding to the effect of doubling the values.
RESULTS
The 280 imatinib plasma concentration values considered ranged between 67 and 11 221 μg l−1. The assessment of AGP plasma concentration in 51 patients (corresponding to 238 samples) provided results ranging from 0.4 to 3.2 g l−1. Among the 38 GIST patients, tumour KIT genotypes of 20 patients were available (corresponding to 111 different plasma samples). Various mutations were detected on the KIT gene: deletions, point mutations or mixed mutations in exon 11 (code=0; n=13), or alternately insertion in exon 9 (AY 502–503 duplication) or wt profile, that is no mutation (code=1; n=7). The patient demographics are presented in Table 1.
Table 1. Patient demographics of the 58 patients evaluated in this concentration–effect analysis (providing 280 plasma samples).
|
Patients
|
Samples
|
|||
|---|---|---|---|---|
| Characteristic | CML | GIST | CML | GIST |
| Pathology diagnosis (no.) | ||||
| GIST | 38 | 227 | ||
| CML | 20 | 53 | ||
| Gender (no.) | ||||
| Men | 9 | 24 | 23 | 138 |
| Women | 11 | 14 | 30 | 89 |
| Age (years) | ||||
| Median | 48 | 57 | ||
| Range | 27–71 | 20–79 | ||
| Imatinib daily dose (mg) | ||||
| Median | 400 | 600 | ||
| Range | 150–800 | 200–800 | ||
| AGP plasma levels (g per l) | ||||
| Median | 0.7 | 0.9 | ||
| Range | 0.4–3.0 | 0.4–3.2 | ||
| KIT genotype (no.) | ||||
| Exon 11 mutation | 13 | 78 | ||
| Exon 9 mutation or wt KIT | 7 | 50 | ||
| Not available | 18 | 99 | ||
| Side effects incidence (no.) | ||||
| 0 | 19 | 52 | ||
| 1 | 15 | 62 | ||
| 2 | 10 | 68 | ||
| 3 or more | 9 | 45 | ||
| Haematological Response score (no.) | ||||
| No response (NHR) | 7 | |||
| Partial response (PHR) | 20 | |||
| Complete response (CHR) | 26 | |||
| RECIST response score (no.) | ||||
| Progressive disease (PD) | 58 | |||
| Stable disease (SD) | 72 | |||
| Partial response (PR) | 87 | |||
| Complete response (CR) | 10 | |||
| Dichotomous response (no.) | ||||
| Progressive or stable disease (SD+PD) | 130 | |||
| Overall responses (OR=PR+CR) | 97 | |||
It is noteworthy that the type of pathology alone was in fact sufficient to predict the response (CML patients had globally better response scores than GIST patients, P<0.001). The results presented below refer to the per-sample analysis. Per-patient analyses gave similar trends, although without reaching significance.
Concentration–effect exploration in CML patients
The pharmacodynamic exploration with total exposure revealed an inverse relationship between Dose, as well as AUC, and therapeutic response (P=0.073 for Dose and P=0.012 for AUC), with non-responding patients receiving higher doses than good responders. Similarly, a better response was associated with higher CL (P=0.023). A similar analysis carried out on toxicity scores showed that Dose and AUC were in turn positively correlated with the amount of side effects, although not significantly (P=0.062 for Dose and P=0.27 for AUC), whereas this was not the case for CL.
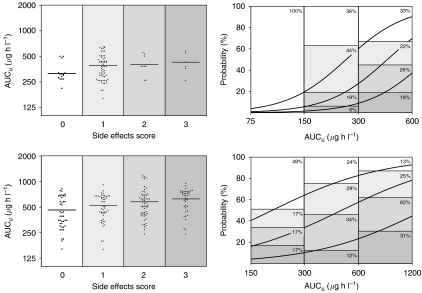
Using free drug exposure estimates (derived from the AGP model previously mentioned) appeared to reverse the relationship between free AUC (AUCu) and response, although not significantly (P=0.48). Furthermore, free clearance (CLu) negatively correlated with the response (P=0.024). Concerning the tolerability to the drug, AUCu remained positively correlated with the amount of side effects (P=0.013). The scatter plot of the upper part of Figure 1 depicts this relationship (left panel) as well as the ordered logistic regression curves (right panel). In the same analysis, CLu also decreased with toxicity scores, although not significantly (P=0.33). The main results of this analysis in CML patients are presented in Table 2.
Figure 1.
Relationship between free drug exposure (AUCu) and toxicity in CML (upper part) and GIST patients (lower part). Left panel: scatter plot of AUCu according to side effects score (0=no side effects, 1=1 side effect, 2=2 side effects and 3=3 or more side effects). Right panel: probability of side effects according to the per-sample PK–PD analyses. The histograms represent the percentages observed for the three types of response at three typical AUCu range values (side effects score: white box=0; light grey box=1; grey box=2; dark grey box=3). The curves, modelled by a four-level ordered logistic regression, show the probability of side effects according to AUCu.
Table 2. Results of the per-sample multivariate logistic regression analysis related to total and free drug exposure.
|
Total exposure
|
||||||||
|---|---|---|---|---|---|---|---|---|
|
All CMLa
|
All GIST
|
Exon 11 mutation GIST
|
Exon 9 mutation or wt GIST
|
|||||
|
53
|
227
|
86
|
36
|
|||||
| n | OR | P-value | OR | P-value | OR | P-value | OR | P-value |
| Response vs | ||||||||
| Dose | 0.3 (±0.2) | 0.073 | 0.7 (±0.2) | 0.206 | 0.6 (±0.2) | 0.306 | → ∞b | 0.008 |
| mut | 0.8 (±0.2) | 0.645 | 1.4 (±0.6) | 0.398 | 0.9 (±0.6) | 0.821 | ||
| AUC | 0.5 (±0.1) | 0.012 | 0.5 (±0.1) | 0.012 | ||||
| AUC | 1.2 (±0.4) | 0.541 | ||||||
| + mut | 0.8 (±0.3) | 0.661 | 0.5 (±0.2) | 0.082 | 8.9 (±8.0) | 0.015 | ||
| CL | 2.6 (±1.1) | 0.023 | 1.5 (±0.4) | 0.097 | ||||
| CL | 1.0 (±0.3) | 0.976 | ||||||
| + mut | 0.8 (±0.3) | 0.646 | ||||||
| Toxicity vs | ||||||||
| Dose | 3.2 (±2.1) | 0.062 | 2.8 (±0.7) | 0.000 | 2.4 (±0.9) | 0.027 | 3.3 (±2.5) | 0.114 |
| mut | 1.9 (±0.8) | 0.071 | 1.2 (±0.4) | 0.544 | 0.5 (±0.3) | 0.224 | ||
| AUC | 1.4 (±0.4) | 0.265 | 2.2 (±0.4) | 0.000 | ||||
| AUC | 1.0 (±0.3) | 0.906 | ||||||
| + mut | 1.9 (±0.7) | 0.071 | 0.5 (±0.3) | 0.147 | 3.2 (±1.7) | 0.032 | ||
| CL | 0.9 (±0.4) | 0.816 | 0.9 (±0.2) | 0.708 | ||||
| CL | 2.0 (±0.6) | 0.017 | ||||||
| + mut | 1.7 (±0.6) | 0.132 | ||||||
|
Free exposure
|
||||||||
|
44
|
193
|
78
|
33
|
|||||
| n | OR | P-value | OR | P-value | OR | P-value | OR | P-value |
| Response vs | ||||||||
| Dose | 0.4 (±0.3) | 0.242 | 0.8 (±0.3) | 0.630 | 0.7 (±0.3) | 0.321 | → ∞b | 0.037c |
| mut | 0.6 (±0.3) | 0.289 | 1.3 (±0.6) | 0.621 | → ∞b | 0.029 | ||
| AUCu | 1.6 (±1.0) | 0.481 | 0.9 (±0.2) | 0.548 | ||||
| AUCu | 2.6 (±1.1) | 0.026 | ||||||
| + mut | 0.6 (±0.2) | 0.299 | 0.1 (±0.1) | 0.007 | 0.1 (±0.1) | 0.104 | ||
| CLu | 0.8 (±0.9) | 0.024 | 1.2 (±0.5) | 0.750 | ||||
| CLu | 0.1 (0.1) | 0.002 | ||||||
| + mut | 1.1 (0.6) | 0.807 | ||||||
| Toxicity vs | ||||||||
| Dose | 7.2 (±6.2) | 0.022 | 2.4 (±0.7) | 0.001 | 2.1 (±0.8) | 0.064 | 4.9 (±4.0) | 0.051 |
| mut | 1.7 (±0.6) | 0.167 | 2.1 (±1.0) | 0.118 | 3.7 (±2.3) | 0.035 | ||
| AUCu | 6.1 (±4.5) | 0.013 | 2.7 (±0.6) | 0.000 | ||||
| AUCu | 2.4 (±0.9) | 0.014 | ||||||
| + mut | 1.7 (±0.6) | 0.148 | 2.4 (±1.8) | 0.240 | 0.2 (±0.2) | 0.154 | ||
| CLu | 0.4 (±0.4) | 0.330 | 0.5 (±0.2) | 0.063 | ||||
| CLu | 1.3 (±0.8) | 0.726 | ||||||
| + mut | 1.6 (±0.6) | 0.241 | ||||||
OR=odds ratios associated with doubling value of the predictor (AUC/AUCu, CL/CLu, Dose) or with mutation profile (mut).
PK parameters expressed as log2 values; response on a 3-point scale for CML and 2-point scale for GIST (OR vs SD+PD), tolerability on a 4-point scale, and dichotomous mutation profile. The odds ratios (± s.e.) represent the effect on efficacy and toxicity score of a doubling of the PK parameter (AUC/AUCu or CL/CLu) or the Dose.
No BCR-ABL mutation detected in the CML population.
Groups entirely distinct.
Approximated value only, due to a lack of sufficient different samples.
Concentration–effect exploration in GIST patients, incorporating KIT genotype
A similar PK–PD analysis incorporating total drug levels in the GIST population again showed some inverse relationship between Dose, AUC or CL and therapeutic response (yet not reaching significance for Dose and CL). This logistic regression analysis also showed that the response tended to be affected by the mutation profile (P=0.071), with patients presenting a resistance-related profile (i.e. KIT exon 9 mutation or wt KIT) showing a lower response rate. In the tolerability analysis, Dose and AUC appeared positively and significantly correlated with the amount of side effects (P<0.001 for Dose and AUC), whereas this was still not the case with CL.
Using free drug exposure estimates (derived from the AGP model) did not change the general relationship between AUCu and response (P=0.63). Concerning the tolerability of the drug, AUCu remained positively correlated with the amount of side effects (P<0.001 in per-sample analysis). Regarding CLu, lower values tended to be associated with lesser side effects, albeit not reaching significance (P=0.063).
Finally, incorporating the genotype profile in the analysis using free level parameters improved to a noticeable degree the relationships previously observed. AUCu indeed correlated with response (P=0.026), whereas CLu appeared inversely linked to response, with lower clearance predicting better outcome (P=0.002). Importantly, AUCu and CLu appeared better predictors of the response than the mutation profile itself (affecting the response, but never significantly in multivariate analyses). Concerning toxicity, AUCu also appeared to be a better predictor than the mutation profile (P=0.014 in multivariate analysis).
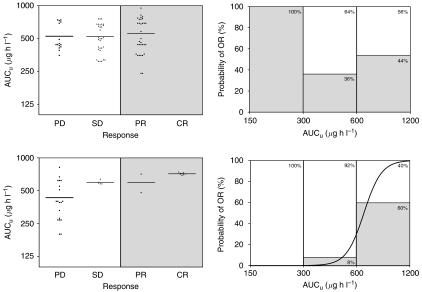
Figure 2 depicts the results of the per-sample concentration–effect analysis with the associated logistic regression curves (probability of response vs AUCu). With exon 11, this curve could not be modelled (no significant differences in response according to AUCu). The histograms represent the percentage of the two types of response at three typical AUCu range values. Table 2 also presents the main results related to this GIST population analysis.
Figure 2.
Relationship between free drug exposure (AUCu) and response in GIST patients. Upper part: exon 11 KIT genotype; lower part: exon 9 or wt KIT genotype. Left panel: scatter plot of AUCu according to RECIST score; white box=PD+SD (score 0; n=23 for exon 9/wt, 46 for exon 11); grey box=OR, OR=CR+PR (score 1; n=10 for exon 9/wt, 32 for exon 11). Right panel: probability of response according to the per-sample PK–PD analysis for both main genotypes of GIST patients. The histograms represent the percentages observed for the two types of response at three typical AUCu range values. The curve, modelled by a two-level ordered logistic regression, shows the probability of response according to AUCu.
DISCUSSION
This clinical exploration reveals that three main confounders can obscure the PK–PD relationship of imatinib: dose selection, protein binding and genetic heterogeneity of the target tumour. Taking into account those three factors allowed observing clearer concentration–response effects.
Several studies had actually suggested that the administration of higher doses than the typical 400 mg daily regimen could improve the response in some patient subsets. A better response was indeed observed in accelerated and blast phases of CML with 600 mg daily (Talpaz et al, 2002), and a 800 mg daily regimen allowed a longer progression-free survival in GIST patients (Verweij et al, 2004), whereas this was not the case with 600 mg (Blanke et al, 2008).
The inverse relationship initially observed in our PK–PD analysis (for both CML and GIST patients) between Dose/AUC and therapeutic response could be considered paradoxical. However, as our study was purely observational, we were in the presence of good responders selected to receive low doses and bad responders high doses, but without apparent advantage. In GIST patients, Dose was indeed highly correlated with AUC and CL, confirming the presumption of such a bias. Conversely, in the CML sub-population, the lower the clearance of the unbound drug, the better was the response, suggesting that CLu was a better predictor of effect than AUC/AUCu. Most CML patients were apparently exposed to sufficient drug amounts to achieve a haematological response (i.e. ceiling of the concentration–effect curve), making them partly obscure the PK–PD relationship. It has indeed been reported that imatinib doses of 350 mg (corresponding to a trough plasma concentration, TPC of 570 μg l−1) already ensure an optimal haematological response in CML (Peng et al, 2004). Such an amount could, however, not be sufficient for a cytogenetic or molecular response, which appears to require TPC as high as 1000 μg l−1 (Picard et al, 2007; Larson et al, 2008).
Moreover, the design of our study wherein AUC derived from sparse measurements were used as an index of exposure may have prevented us from observing similar results as in the IRIS study (steady-state imatinib TPC at initiation of therapy in patients on 400 mg QD predicts long-term complete cytogenetic and major molecular responses) (Larson et al, 2008). As the PK–PD relationship for a targeted agent such as imatinib may be confounded by genotypic heterogeneity of intracellular pharmacological targets (BCR-ABL and c-KIT, respectively), the mutational status of BCR-ABL was also assessed in our CML population by DNA sequencing. However, no point mutations known to confer resistance were observed (data not shown).
Conversely, focusing on GISTs allowed us to uncover a relationship between free drug exposure and response when integrating the target mutation profile (with higher drug exposure predicting better response, and being a superior predictor than the mutation status). Of importance, the inclusion of SD in the OR score did not significantly affect the correlations observed. Imatinib-free plasma levels thus appeared a better predictor of drug effect than total levels. This is in line with previous data showing that the total plasma concentration of imatinib is a poor marker of imatinib clinical effect (Delbaldo et al, 2006). Very recently, however, Demetri presented data showing that imatinib total TPC could correlate with response (expressed also as OR=CR+PR) in a larger GIST population, and this more significantly than AUC (Demetri et al, 2008).
On the basis of our data, Figure 2 suggests that patients with tumours harbouring a ‘sensitive’ c-KIT genotype (KIT exon 11 mutations) are exposed to concentrations that are already near the top of the concentration–response curve (as was probably the case in our CML patients; see above). On the other hand, patients with a ‘resistant’ genotype (exon 9 mutations or wt KIT) are probably lying in the steep part of the curve, where a definite concentration–response relationship can be observed. Such patients could probably draw the most benefit from a thorough adjustment of their imatinib exposure. It has indeed been demonstrated that patients harbouring an exon 9 mutation benefit the most from a 800 mg daily regimen (Debiec-Rychter et al, 2006). When taking into account the mutation profile in our analysis, lower CLu also proved to predict better responses in both groups. Again, the poor correlation between concentration and response observed without considering the mutation profile suggests that this relationship could be obscured by a Dose selection effect.
Our study has thus been able to demonstrate for the first time a clear relationship between exposure to the unbound drug and clinical efficacy of imatinib in GIST patients. It provides a clinically relevant PK–PD model using logistic regression with formal assessment of in vivo concentration–effect curves, instead of a mere comparison of PK parameters (e.g. TPC) between responders and non-responders. Additionally, our PK–PD exploration formally established that the occurrence of side effects is more frequent at higher imatinib exposure levels (Figure 1). Together with previous data (Delbaldo et al, 2006), this indicates that monitoring imatinib plasma levels may help to identify patients with unnecessarily high levels at risk of developing toxicity. In the literature, several cases have indeed been reported where imatinib treatment had to be discontinued because of the occurrence of serious adverse events (Brouard and Saurat, 2001; Elliott et al, 2002; Gambillara et al, 2005; Blasdel et al, 2007). In some cases, plasma drug measurement and dose adjustment were considered (Blasdel et al, 2007; Gambillara et al, 2005). Concerning our data, it is worth noting, however, that a severity scale should have been used (typically NCI-CTC). As mentioned above, it was not available at the time of our study. The incidence scale used instead has been applied elsewhere (Schuell et al, 2005), but it has to be considered cautiously and may prevent formal comparison with other studies. It, however, allowed a general delineation of concentration–toxicity relationships.
Our exploratory study (performed on a small patient set), associated to data already published for CML (Picard et al, 2007; Larson et al, 2008) and for GIST (Demetri et al, 2008), should thus stimulate further confirmation in larger populations of the relationship between imatinib exposure, suitably free plasma level, and its efficacy and toxicity. A prospective study to validate a therapeutic drug monitoring (TDM) approach is indeed being initiated in France (Picard et al, 2007). Such paradigms will potentially apply to other new targeted anticancer drugs under development or already approved by registration authorities. For instance, it has recently been shown in an animal model that tumoural phospho-BCR-ABL inhibition is directly correlated with plasma levels of dasatinib, a novel BCR-ABL inhibitor (Luo et al, 2006). For imatinib, the additional monitoring of the active N-demethylated metabolite may also be considered (Delbaldo et al, 2006). Our data also suggest that patient stratification by genotype will be important for future investigation. As recently stated, molecular subclassification is becoming an important element for providing personalised care to oncologic patients (Heinrich and Corless, 2006).
In conclusion, the various PK–PD relationships progressively uncovered, together with some case reports on the benefit of such an approach in imatinib treated patients (Blasdel et al, 2007), provide arguments to evaluate further the potential benefit of a TDM programme in well-controlled clinical trials. As recently declared by Brian Druker (quoted in Tuma, 2007), targeted anticancer drugs treatment may follow the HIV model, notably by combination therapy (see also Stebbing and Bower, 2003). In HIV patients, TDM is increasingly recommended (e.g. for drug interactions, in case of toxicity and for drug exposure assessment) in association with the viral genotype profile. Therefore, in oncology, an approach that integrates clinical PKs and patient/tumour pharmacogenetics may well contribute to optimise the therapeutic use of new drugs, such as signal transduction inhibitors, in patients.
Acknowledgments
Investigators initiated the study, supported in part by the Programme of the Master of Advanced Studies in Hospital Pharmacy (Professor A Pannatier, Service of Pharmacy, University Hospital Centre, Lausanne).
References
- Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, Leversha MA, Jeffrey PD, Desantis D, Singer S, Brennan MF, Maki RG, DeMatteo RP (2005) Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 11: 4182–4190 [DOI] [PubMed] [Google Scholar]
- Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D, Wehre E, Nikolova Z, Joensuu H (2008) Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26: 620–625 [DOI] [PubMed] [Google Scholar]
- Blasdel C, Egorin MJ, Lagattuta TF, Druker BJ, Deininger MW (2007) Therapeutic drug monitoring in CML patients on imatinib. Blood 110: 1699–1701; author reply 1701 [DOI] [PubMed] [Google Scholar]
- Brouard M, Saurat JH (2001) Cutaneous reactions to STI571. N Engl J Med 345: 618–619 [DOI] [PubMed] [Google Scholar]
- Capdeville R, Buchdunger E, Zimmermann J, Matter A (2002) Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov 1: 493–502 [DOI] [PubMed] [Google Scholar]
- Cohen MH, Johnson JR, Pazdur R (2005) US Food and Drug Administration drug approval summary: conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin Cancer Res 11: 12–19 [PubMed] [Google Scholar]
- Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE (2005) hOCT1 and resistance to imatinib. Blood 106: 1133–1134; author reply 1134 [DOI] [PubMed] [Google Scholar]
- Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, Dimitrijevic S, Sciot R, Stul M, Vranck H, Scurr M, Hagemeijer A, van Glabbeke M, van Oosterom AT, Eortc Soft Tissue and Bone Sarcoma Group (2004) Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 40: 689–695 [DOI] [PubMed] [Google Scholar]
- Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, Blay J-Y, Leyvraz S, Stul M, Casali PG (2006) KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer 42: 1093–1103 [DOI] [PubMed] [Google Scholar]
- Delbaldo C, Chatelut E, Re M, Deroussent A, Seronie-Vivien S, Jambu A, Berthaud P, Le Cesne A, Blay JY, Vassal G (2006) Pharmacokinetic–pharmacodynamic relationships of imatinib and its main metabolite in patients with advanced gastrointestinal stromal tumors. Clin Cancer Res 12: 6073–6078 [DOI] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347: 472–480 [DOI] [PubMed] [Google Scholar]
- Demetri GD, Wang Y, Wehrle E, Blanke C, Joensuu H, von Mehren M (2008) Correlation of imatinib plasma levels with clinical benefit in patients (Pts) with unresectable/metastatic gastrointestinal stromal tumors (GIST) (abstract, oral presentation). In 2008 Gastrointestinal Cancers Symposium. Orlando, January 25–27, 2008 [Google Scholar]
- Druker BJ (2003) Imatinib mesylate in the treatment of chronic myeloid leukaemia. Expert Opin Pharmacother 4: 963–971 [DOI] [PubMed] [Google Scholar]
- Elliott MA, Mesa RA, Tefferi A (2002) Adverse events after imatinib mesylate therapy. N Engl J Med 346: 712–713 [DOI] [PubMed] [Google Scholar]
- Gambacorti-Passerini C, Barni R, le Coutre P, Zucchetti M, Cabrita G, Cleris L, Rossi F, Gianazza E, Brueggen J, Cozens R, Pioltelli P, Pogliani E, Corneo G, Formelli F, D'Incalci M (2000) Role of alpha1 acid glycoprotein in the in vivo resistance of human BCR-ABL(+) leukemic cells to the abl inhibitor STI571. J Natl Cancer Inst 92: 1641–1650 [DOI] [PubMed] [Google Scholar]
- Gambacorti-Passerini C, Zucchetti M, Russo D, Frapolli R, Verga M, Bungaro S, Tornaghi L, Rossi F, Pioltelli P, Pogliani E, Alberti D, Corneo G, D'Incalci M (2003) Alpha1 acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients. Clin Cancer Res 9: 625–632 [PubMed] [Google Scholar]
- Gambillara E, Laffitte E, Widmer N, Decosterd LA, Duchosal MA, Kovacsovics T, Panizzon RG (2005) Severe pustular eruption associated with imatinib and voriconazole in a patient with chronic myeloid leukemia. Dermatology 211: 363–365 [DOI] [PubMed] [Google Scholar]
- Gibbons J, Egorin MJ, Ramanathan RK, Fu P, Mulkerin DL, Shibata S, Takimoto CH, Mani S, LoRusso PA, Grem JL, Pavlick A, Lenz HJ, Flick SM, Reynolds S, Lagattuta TF, Parise RA, Wang Y, Murgo AJ, Ivy SP, Remick SC (2008) Phase I and pharmacokinetic study of imatinib mesylate in patients with advanced malignancies and varying degrees of renal dysfunction: a study by the National Cancer Institute Organ Dysfunction Working Group. J Clin Oncol 26: 570–576 [DOI] [PubMed] [Google Scholar]
- Gurney H, Wong M, Balleine RL, Rivory LP, McLachlan AJ, Hoskins JM, Wilcken N, Clarke CL, Mann GJ, Collins M, Delforce SE, Lynch K, Schran H (2007) Imatinib disposition and ABCB1 (MDR1, P-glycoprotein) genotype. Clin Pharmacol Ther 82: 33–40 [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL (2006) Does tumor mutational status correlate with clinical response to imatinib? Nat Clin Pract Oncol 3: 600–601 [DOI] [PubMed] [Google Scholar]
- Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, Singer S, Fletcher CD, Silberman S, Dimitrijevic S, Fletcher JA (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21: 4342–4349 [DOI] [PubMed] [Google Scholar]
- Hochhaus A, La Rosee P (2004) Imatinib therapy in chronic myelogenous leukemia: strategies to avoid and overcome resistance. Leukemia 18: 1321–1331 [DOI] [PubMed] [Google Scholar]
- Judson I, Peiming M, Peng B, Verweij J, Racine A, di Paola ED, van Glabbeke M, Dimitrijevic S, Scurr M, Dumez H, van Oosterom A (2005) Imatinib pharmacokinetics in patients with gastrointestinal stromal tumour: a retrospective population pharmacokinetic study over time. EORTC Soft Tissue and Bone Sarcoma Group. Cancer Chemother Pharmacol 55: 379–386 [DOI] [PubMed] [Google Scholar]
- Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand J-B, Force T (2006) Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 12: 908–916 [DOI] [PubMed] [Google Scholar]
- Larghero J, Leguay T, Mourah S, Madelaine-Chambrin I, Taksin AL, Raffoux E, Bastie JN, Degos L, Berthaud P, Marolleau JP, Calvo F, Chomienne C, Mahon FX, Rousselot P (2003) Relationship between elevated levels of the alpha 1 acid glycoprotein in chronic myelogenous leukemia in blast crisis and pharmacological resistance to imatinib (Gleevec) in vitro and in vivo. Biochem Pharmacol 66: 1907–1913 [DOI] [PubMed] [Google Scholar]
- Larson RA, Druker BJ, Guilhot FA, O'Brien SG, Riviere GJ, Krahnke T, Gathmann I, Wang Y (2008) Imatinib pharmacokinetics and its correlation with response and safety in chronic phase chronic myeloid leukemia: a subanalysis of the IRIS study (prepublished online February 6, 2008). Blood, doi:10.1182/blood-2007-10-116475 [DOI] [PubMed]
- Luo FR, Yang Z, Camuso A, Smykla R, McGlinchey K, Fager K, Flefleh C, Castaneda S, Inigo I, Kan D, Wen ML, Kramer R, Blackwood-Chirchir A, Lee FY (2006) Dasatinib (BMS-354825) pharmacokinetics and pharmacodynamic biomarkers in animal models predict optimal clinical exposure. Clin Cancer Res 12: 7180–7186 [DOI] [PubMed] [Google Scholar]
- Mahon FX, Belloc F, Lagarde V, Chollet C, Moreau-Gaudry F, Reiffers J, Goldman JM, Melo JV (2003) MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood 101: 2368–2373 [DOI] [PubMed] [Google Scholar]
- Michor F (2007) Reply: The long-term response to imatinib treatment of CML. Br J Cancer 96: 679–680 [Google Scholar]
- Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA (2005) Dynamics of chronic myeloid leukaemia. Nature 435: 1267–1270 [DOI] [PubMed] [Google Scholar]
- Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, Sawyers CL, Rosamilia M, Ford J, Lloyd P, Capdeville R (2004) Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 22: 935–942 [DOI] [PubMed] [Google Scholar]
- Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, Lassalle R, Marit G, Reiffers J, Begaud B, Moore N, Molimard M, Mahon FX (2007) Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 109: 3496–3499 [DOI] [PubMed] [Google Scholar]
- Ramanathan RK, Egorin MJ, Takimoto CH, Remick SC, Doroshow JH, LoRusso PA, Mulkerin DL, Grem JL, Hamilton A, Murgo AJ, Potter DM, Belani CP, Hayes MJ, Peng B, Ivy SP (2008) Phase I and pharmacokinetic study of imatinib mesylate in patients with advanced malignancies and varying degrees of liver dysfunction: a study by the National Cancer Institute Organ Dysfunction Working Group. J Clin Oncol 26: 563–569 [DOI] [PubMed] [Google Scholar]
- Rochat B, Fayet A, Widmer N, Lahrichi SL, Pesse B, Décosterd LA, Biollaz J (2008) Imatinib metabolite profiling in parallel to imatinib quantification in plasma of treated patients using liquid chromatography-mass spectrometry (prepublished online on February 20, 2008). J Mass Spectrom, doi:10.1002/jms.1369 [DOI] [PubMed]
- Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, Schiffer CA, Talpaz M, Guilhot F, Deininger MW, Fischer T, O'Brien SG, Stone RM, Gambacorti-Passerini CB, Russell NH, Reiffers JJ, Shea TC, Chapuis B, Coutre S, Tura S, Morra E, Larson RA, Saven A, Peschel C, Gratwohl A, Mandelli F, Ben-Am M, Gathmann I, Capdeville R, Paquette RL, Druker BJ (2002) Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood 99: 3530–3539 [DOI] [PubMed] [Google Scholar]
- Schmidli H, Peng B, Riviere GJ, Capdeville R, Hensley M, Gathmann I, Bolton AE, Racine-Poon A (2005) Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol 60: 35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuell B, Gruenberger T, Kornek GV, Dworan N, Depisch D, Lang F, Schneeweiss B, Scheithauer W (2005) Side effects during chemotherapy predict tumour response in advanced colorectal cancer. Br J Cancer 93: 744–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsson T, Sjolund K, Bumming P, Ahlman H, Nilsson B, Oden A (2007) Reducing uncertainty in health-care resource allocation. Br J Cancer 96: 1834–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stata Corp (2003) Stata Base Reference Manual, Vol. 3, 8th edn, Stata Press: College Station, TX [Google Scholar]
- Stebbing J, Bower M (2003) What can oncologists learn from HIV? Lancet Oncol 4: 438–445 [DOI] [PubMed] [Google Scholar]
- Steinert DM, McAuliffe JC, Trent JC (2005) Imatinib mesylate in the treatment of gastrointestinal stromal tumour. Expert Opin Pharmacother 6: 105–113 [DOI] [PubMed] [Google Scholar]
- Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, Schiffer CA, Fischer T, Deininger MW, Lennard AL, Hochhaus A, Ottmann OG, Gratwohl A, Baccarani M, Stone R, Tura S, Mahon FX, Fernandes-Reese S, Gathmann I, Capdeville R, Kantarjian HM, Sawyers CL (2002) Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood 99: 1928–1937 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Thomas J, Wang L, Clark RE, Pirmohamed M (2004) Active transport of imatinib into and out of cells: implications for drug resistance. Blood 104: 3739–3745 [DOI] [PubMed] [Google Scholar]
- Tothova E, Kafkova A, Fricova M, Benova B, Kirschnerova G, Tothova A (2005) Imatinib mesylate in Philadelphia chromosome-positive, chronic-phase myeloid leukemia after failure of interferon alpha. Neoplasma 52: 63–67 [PubMed] [Google Scholar]
- Tsang J, Rudychev I, Pescatore SL (2006) Prescription compliance and persistency in chronic myelogenous leukemia (CML) and gastrointestinal stromal tumor (GIST) patients (pts) on imatinib (IM). J Clin Oncol ASCO Annual Meeting Proceedings Part I. 24: 6119 [Google Scholar]
- Tuma RS (2007) With targeted drugs, chronic myelogenous leukemia therapy may follow HIV's model. J Natl Cancer Inst 99: 192–194 [DOI] [PubMed] [Google Scholar]
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I (2004) Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 364: 1127–1134 [DOI] [PubMed] [Google Scholar]
- Wang L, Giannoudis A, Lane S, Williamson P, Pirmohamed M, Clark RE (2008) Expression of the uptake drug transporter hOCT1 is an important clinical determinant of the response to imatinib in chronic myeloid leukemia. Clin Pharmacol Ther 83: 258–264 [DOI] [PubMed] [Google Scholar]
- Weisberg E, Griffin JD (2003) Resistance to imatinib (Glivec): update on clinical mechanisms. Drug Resist Updat 6: 231–238 [DOI] [PubMed] [Google Scholar]
- Widmer N, Béguin A, Rochat B, Buclin T, Kovacsovics T, Duchosal MA, Leyvraz S, Rosselet A, Biollaz J, Decosterd LA (2004) Determination of imatinib (Gleevec) in human plasma by solid-phase extraction-liquid chromatography-ultraviolet absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci 803: 285–292 [DOI] [PubMed] [Google Scholar]
- Widmer N, Decosterd LA, Csajka C, Leyvraz S, Duchosal MA, Rosselet A, Rochat B, Eap CB, Henry H, Biollaz J, Buclin T (2006) Population pharmacokinetics of imatinib and role of alpha-1-acid glycoprotein. Br J Clin Pharmacol 62: 97–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer N, Rumpold H, Untergasser G, Fayet A, Buclin T, Decosterd LA (2007) Resistance reversal by RNAi silencing of MDR1 in CML cells associated with increase in imatinib intracellular levels. Leukemia 21: 1561–1562; author reply 1562–1564 [DOI] [PubMed] [Google Scholar]
- Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, Schlemmer M, Van Glabbeke M, Brown M, Judson IR, EORTC Soft Tissue and Bone Sarcoma Group – the Italian Sarcoma Group; Australasian Gastrointestinal Trials Group (2005) Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer 41: 1751–1757 [DOI] [PubMed] [Google Scholar]