Abstract
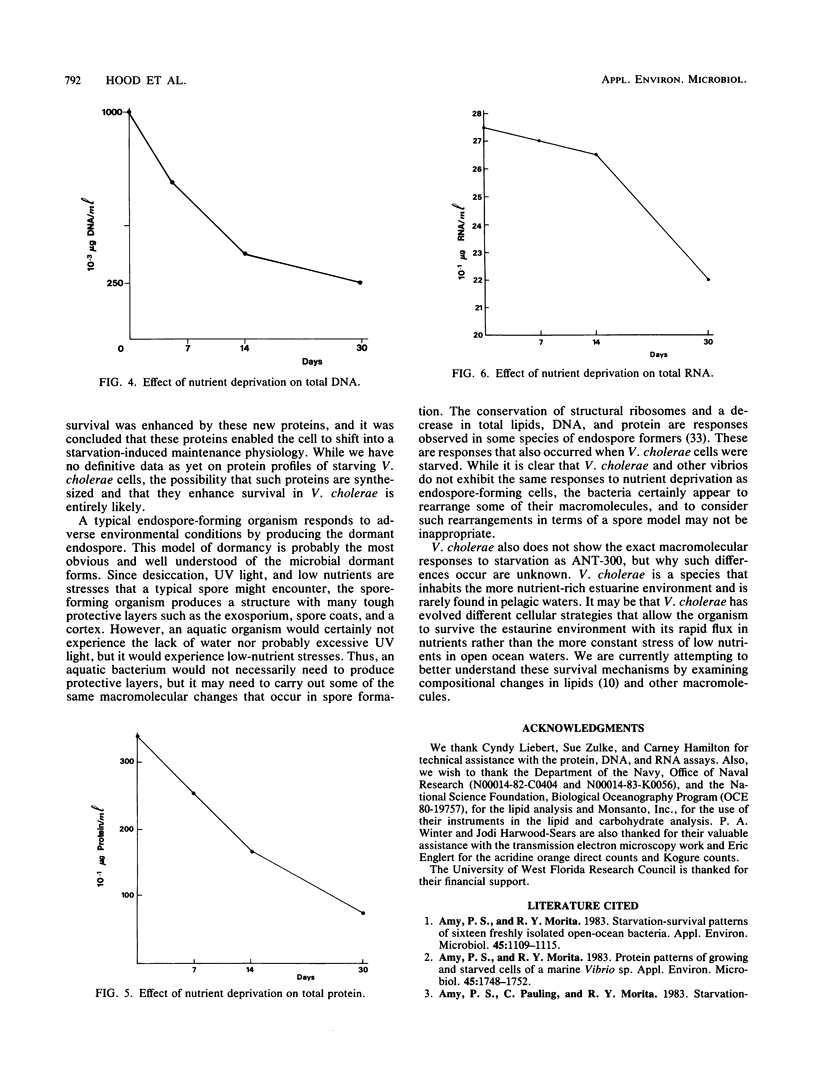
The response of Vibrio cholerae to low nutrient levels was determined by measuring the concentrations of lipids, carbohydrates, DNA, RNA, and proteins over a 30-day starvation period. Ultrastructural integrity was observed by transmission electron microscopy. Total lipids and carbohydrates declined rapidly within the first 7 days, while DNA and protein exhibited a more constant decline over the 30 days of starvation. In contrast, RNA showed little decrease upon starvation. Although neutral lipids were lost, the percentage of neutral lipids did not decline as rapidly as the phospholipids. Detectable levels of poly-beta-hydroxybutyrate disappeared completely by 7 days. Carbohydrate profiles revealed the relative loss of the five-carbon sugar ribose and N-acetylglucosamine and a relative increase in the total six-carbon sugars, especially glucose. Morphologically, ribosomes appeared to exhibit no structural change, while inclusion bodies and mesosomelike structures disappeared completely, and cell wall and membrane integrity was lost. The data suggest that V. cholerae differs somewhat from other marine vibrios in its response to low nutrients but shares some characteristics in common with them. The data also suggest that certain lipids and carbohydrates may provide the endogenous energy sources needed for dormancy preparation and cell maintenance under nutrient starvation.
Full text
PDF
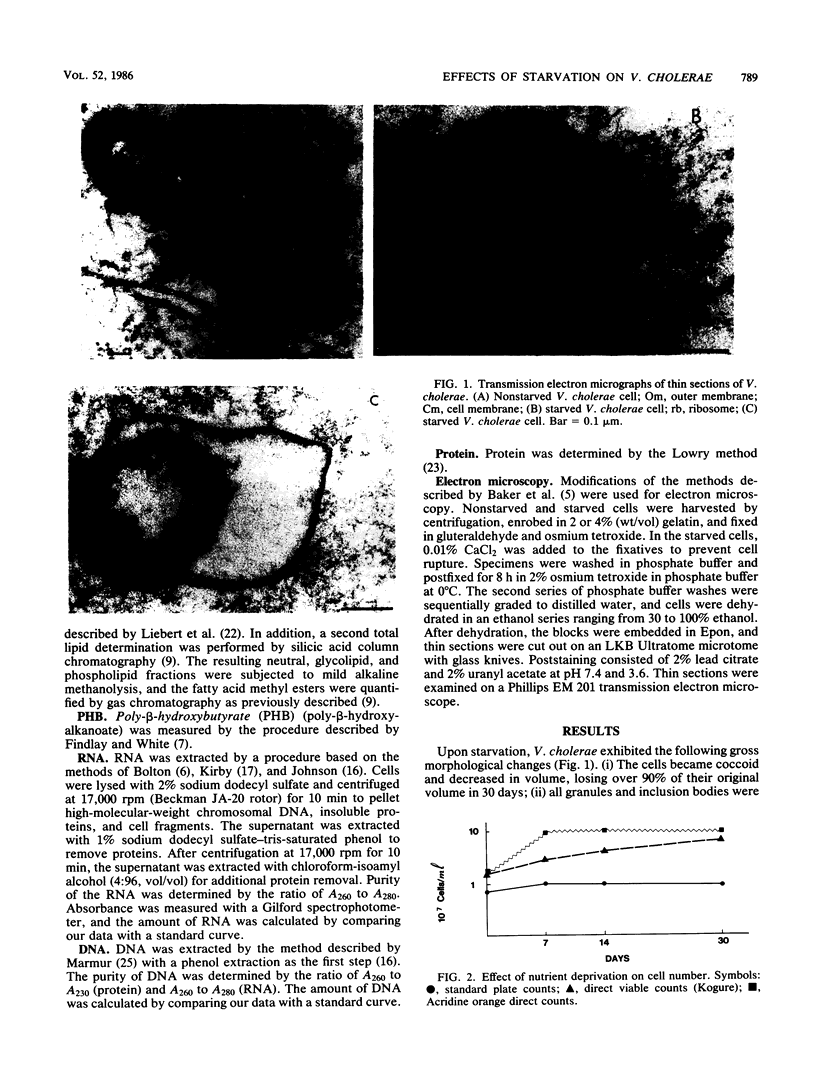
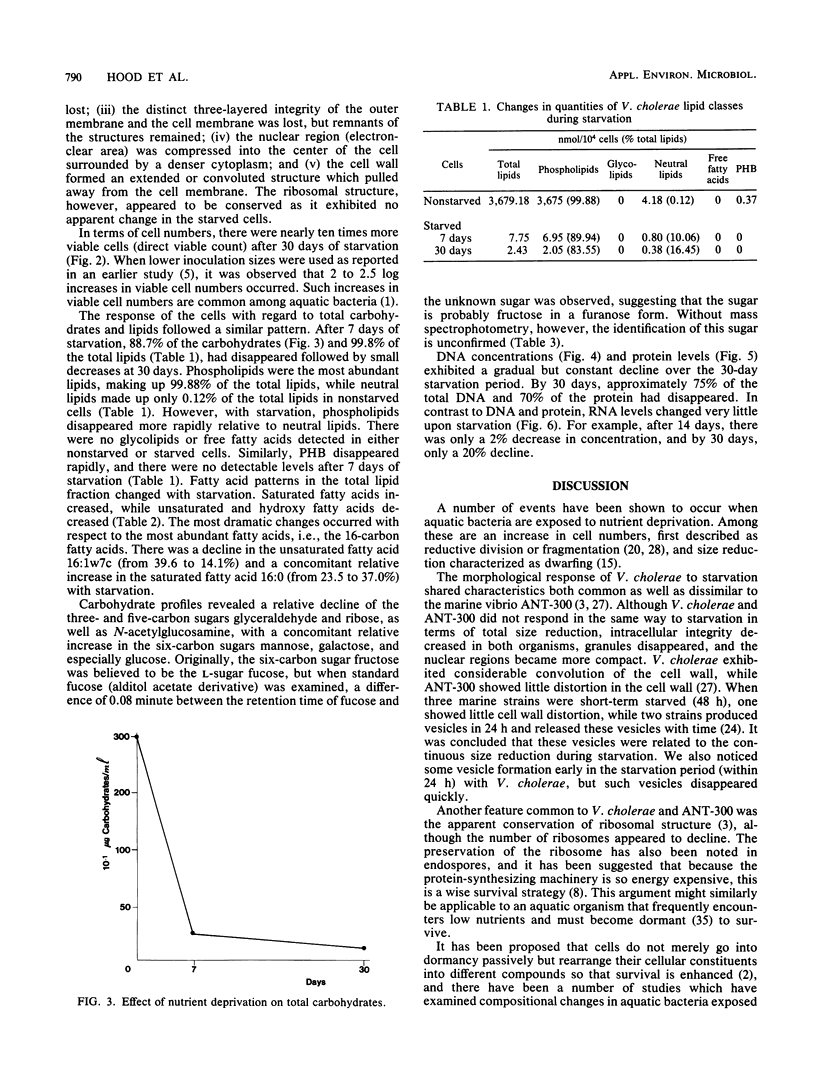
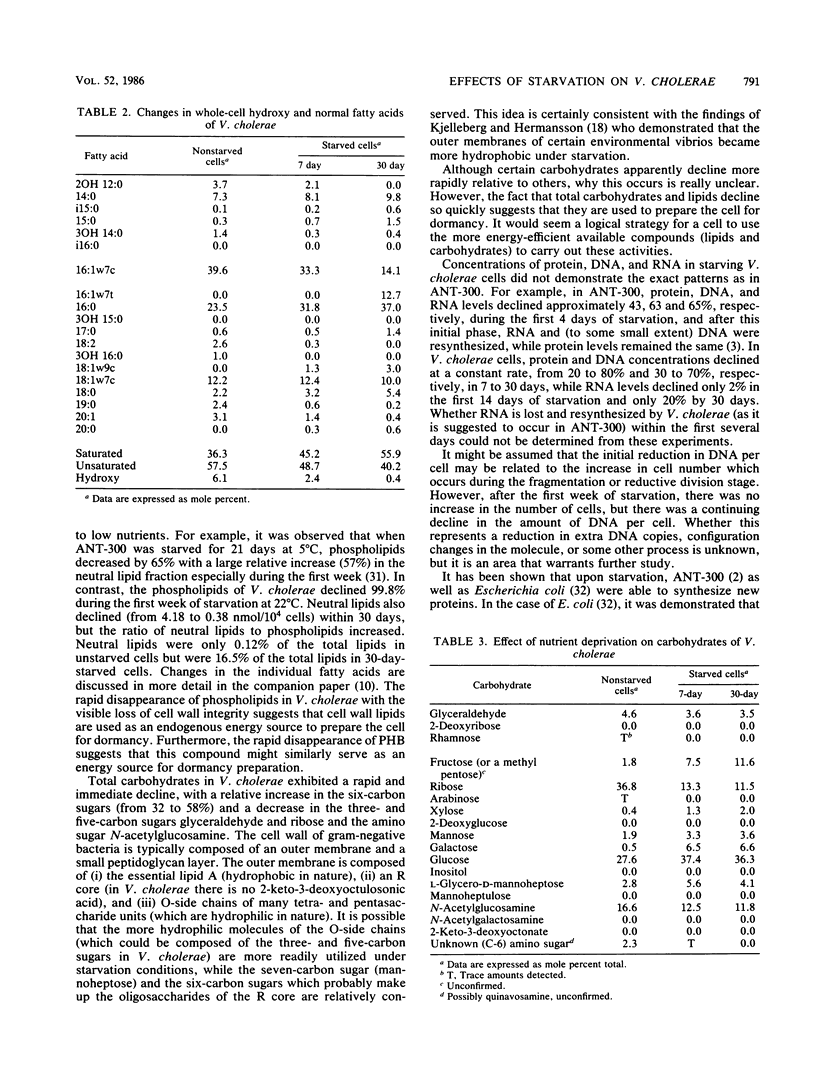

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Morita R. Y. Protein Patterns of Growing and Starved Cells of a Marine Vibrio sp. Appl Environ Microbiol. 1983 Jun;45(6):1748–1752. doi: 10.1128/aem.45.6.1748-1752.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Morita R. Y. Starvation-survival patterns of sixteen freshly isolated open-ocean bacteria. Appl Environ Microbiol. 1983 Mar;45(3):1109–1115. doi: 10.1128/aem.45.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Pauling C., Morita R. Y. Recovery from nutrient starvation by a marine Vibrio sp. Appl Environ Microbiol. 1983 May;45(5):1685–1690. doi: 10.1128/aem.45.5.1685-1690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Pauling C., Morita R. Y. Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol. 1983 Mar;45(3):1041–1048. doi: 10.1128/aem.45.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. M., Singleton F. L., Hood M. A. Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol. 1983 Oct;46(4):930–940. doi: 10.1128/aem.46.4.930-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay R. H., White D. C. Polymeric Beta-Hydroxyalkanoates from Environmental Samples and Bacillus megaterium. Appl Environ Microbiol. 1983 Jan;45(1):71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guckert J. B., Hood M. A., White D. C. Phospholipid ester-linked fatty acid profile changes during nutrient deprivation of Vibrio cholerae: increases in the trans/cis ratio and proportions of cyclopropyl fatty acids. Appl Environ Microbiol. 1986 Oct;52(4):794–801. doi: 10.1128/aem.52.4.794-801.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder W., Dijkhuizen L. Physiological responses to nutrient limitation. Annu Rev Microbiol. 1983;37:1–23. doi: 10.1146/annurev.mi.37.100183.000245. [DOI] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. A., Ness G. E. Survival of Vibrio cholerae and Escherichia coli in estuarine waters and sediments. Appl Environ Microbiol. 1982 Mar;43(3):578–584. doi: 10.1128/aem.43.3.578-584.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey B., Kjelleberg S., Marshall K. C. Responses of marine bacteria under starvation conditions at a solid-water interface. Appl Environ Microbiol. 1983 Jan;45(1):43–47. doi: 10.1128/aem.45.1.43-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M. Starvation-induced effects on bacterial surface characteristics. Appl Environ Microbiol. 1984 Sep;48(3):497–503. doi: 10.1128/aem.48.3.497-503.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Effect of interfaces on small, starved marine bacteria. Appl Environ Microbiol. 1982 May;43(5):1166–1172. doi: 10.1128/aem.43.5.1166-1172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Initial phases of starvation and activity of bacteria at surfaces. Appl Environ Microbiol. 1983 Nov;46(5):978–984. doi: 10.1128/aem.46.5.978-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liebert C. A., Hood M. A., Deck F. H., Bishop K., Flaherty D. K. Isolation and characterization of a new Cytophaga species implicated in a work-related lung disease. Appl Environ Microbiol. 1984 Nov;48(5):936–943. doi: 10.1128/aem.48.5.936-943.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Morphological characterization of small cells resulting from nutrient starvation of a psychrophilic marine vibrio. Appl Environ Microbiol. 1976 Oct;32(4):617–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky J. A., Morita R. Y. Survival of a psychrophilic marine Vibrio under long-term nutrient starvation. Appl Environ Microbiol. 1977 Mar;33(3):635–641. doi: 10.1128/aem.33.3.635-641.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades J. M. Gas-liquid chromatography of alditol acetates and its application to the analysis of sugars in complex hydrolysates. J Chromatogr. 1967 Jun;28(2):246–252. doi: 10.1016/s0021-9673(01)85963-x. [DOI] [PubMed] [Google Scholar]
- Oliver J. D., Stringer W. F. Lipid Composition of a Psychrophilic Marine Vibrio sp. During Starvation-Induced Morphogenesis. Appl Environ Microbiol. 1984 Mar;47(3):461–466. doi: 10.1128/aem.47.3.461-466.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve C. A., Amy P. S., Matin A. Role of protein synthesis in the survival of carbon-starved Escherichia coli K-12. J Bacteriol. 1984 Dec;160(3):1041–1046. doi: 10.1128/jb.160.3.1041-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]