Abstract
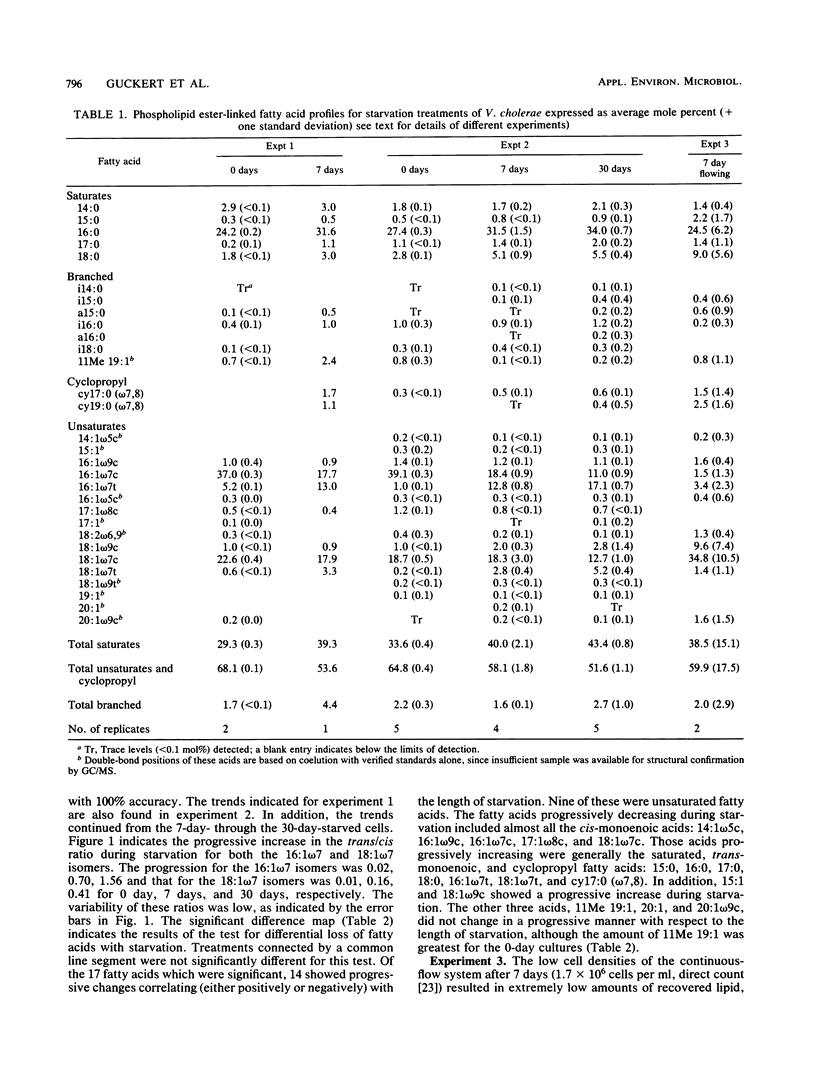
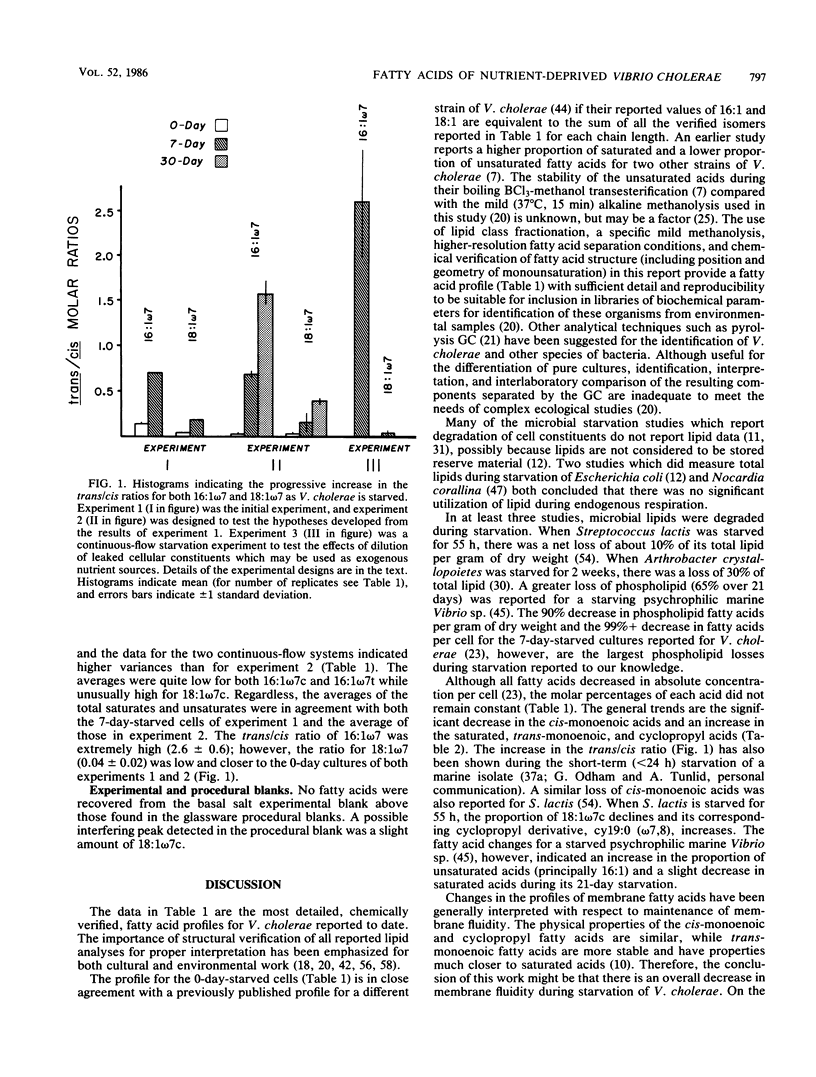
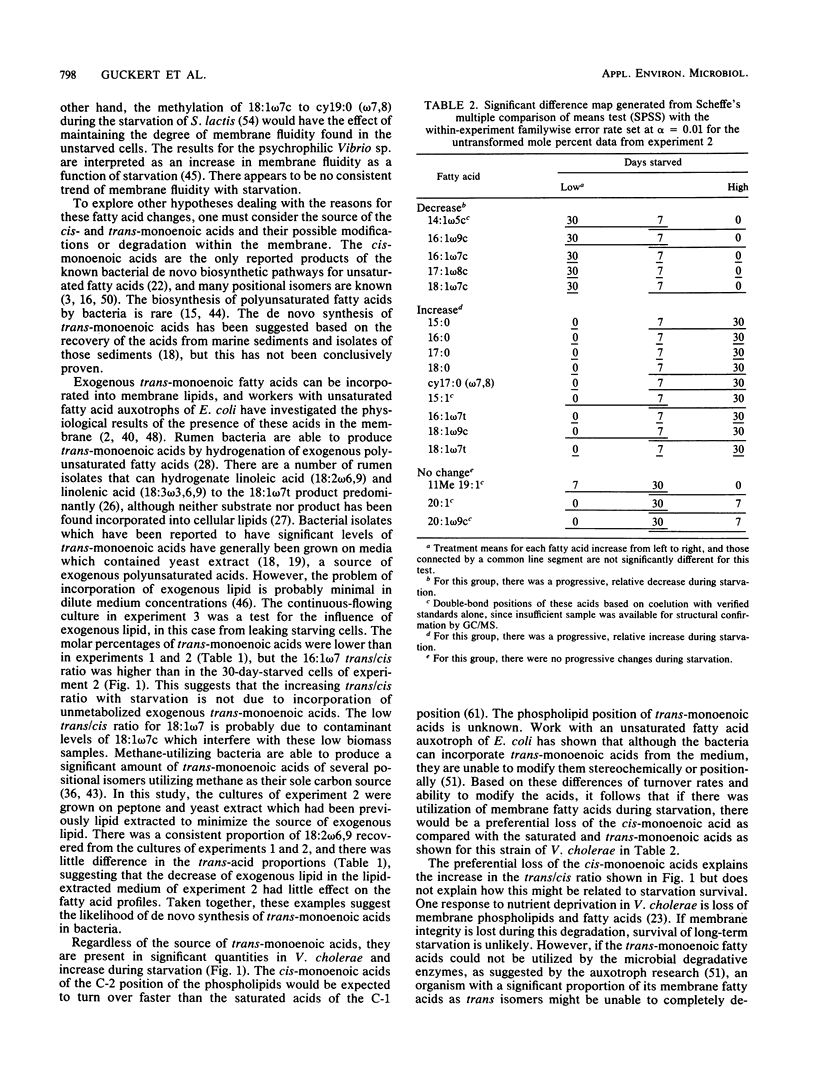
The phospholipid ester-linked fatty acids of 0-day-, 7-day-, and 30-day-starved cultures of Vibrio cholerae were compared. Statistically significant trends were noted in the fatty acid profiles as the cells starved. The amount of the cis-monoenoic fatty acids declined (e.g., 16:1 omega 7c: 0 day, 39%; 7 day, 18%; 30 day, 11%). In contrast, the saturated fatty acids, the cyclopropyl derivatives of the cis-monoenoic fatty acids, and trans-monoenoic fatty acids increased during starvation. For instance, the amounts of 16:1 omega 7t were: 0 day, 1%; 7 day, 13%; 30 day, 17%; which increased the trans/cis ratio for 16:1 omega 7 from 0.02 (0 day) to 0.70 (7 day) to 1.56 (30 day). This may be due to the reported high turnover rates of cis-monoenoic fatty acids of membrane phospholipids and the availability of enzymes for the metabolism of these isomers. During starvation-induced phospholipid loss, the cis-monoenoic fatty acids would, therefore, be preferentially utilized. The ability to either synthesize trans-monoenoic acids (which are not easily metabolized by bacteria) or modify the more volatile cis-monoenoic acids to their cyclopropyl derivatives may be a survival mechanism which helps maintain a functional (although structurally altered) membrane during starvation-induced lipid utilization. In addition, a trans/cis fatty acid ratio significantly greater than that reported for most bacterial cultures and environmental samples (less than 0.1) may be used as a starvation or stress lipid index. Such a ratio could help determine the nutritional status of ultramicrobacteria and other reported dormant cells in natural aquatic environments.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH K., BARONOWSKY P., GOLDFINE H., LENNARZ W. J., LIGHT R., NORRIS A. T., SCHEUERBRANDT G. Biosynthesis and metabolism of unsaturated fatty acids. Fed Proc. 1961 Dec;20:921–927. [PubMed] [Google Scholar]
- Baker R. M., Singleton F. L., Hood M. A. Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol. 1983 Oct;46(4):930–940. doi: 10.1128/aem.46.4.930-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham I. R., Silbert D. F. Studies on the uridine diphosphate-galactose: lipopolysaccharide galactosyltransferase reaction using a fatty acid mutant of Escherichia coli. J Biol Chem. 1973 Aug 10;248(15):5310–5318. [PubMed] [Google Scholar]
- Brian B. L., Gardner E. W. Cyclopropane fatty acids of rugose Vibrio cholerae. J Bacteriol. 1968 Dec;96(6):2181–2182. doi: 10.1128/jb.96.6.2181-2182.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian B. L., Gardner E. W. Fatty acids from Vibrio cholerae lipids. J Infect Dis. 1968 Feb;118(1):47–53. doi: 10.1093/infdis/118.1.47. [DOI] [PubMed] [Google Scholar]
- Bøe B., Gjerde J. Fatty acid patterns in the classification of some representatives of the families Enterobacteriaceae and Vibrionaceae. J Gen Microbiol. 1980 Jan;116(1):41–49. doi: 10.1099/00221287-116-1-41. [DOI] [PubMed] [Google Scholar]
- Chapman D., Owens N. F., Walker D. A. Physical studies of phospholipids. II. Monolayer studies of some synthetic 2,3-diacyl-DL-phosphatidylethanolamines and phosphatidylcholines containing trans double bonds. Biochim Biophys Acta. 1966 May 12;120(1):148–155. doi: 10.1016/0926-6585(66)90286-x. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes E. A., Large P. J. Effect of starvation on the viability and cellular constituents of Zymomonas anaerobia and Zymomonas mobilis. J Gen Microbiol. 1970 Jan;60(1):31–42. doi: 10.1099/00221287-60-1-31. [DOI] [PubMed] [Google Scholar]
- Drucker D. B. Chemotaxonomic fatty-acid fingerprints of some streptococci with subsequent statistical analysis. Can J Microbiol. 1974 Dec;20(12):1723–1728. doi: 10.1139/m74-266. [DOI] [PubMed] [Google Scholar]
- FULCO A. J., LEVY R., BLOCH K. THE BIOSYNTHESIS OF DELTA-9 AND DELTA-5-MONOSATURATED FATTY ACIDS BY BACTERIA. J Biol Chem. 1964 Apr;239:998–1003. [PubMed] [Google Scholar]
- Fulco A. J. Metabolic alterations of fatty acids. Annu Rev Biochem. 1974;43(0):215–241. doi: 10.1146/annurev.bi.43.070174.001243. [DOI] [PubMed] [Google Scholar]
- Gillan F. T., Johns R. B., Verheyen T. V., Volkman J. K., Bavor H. J. trans-Monounsaturated Acids in a Marine Bacterial Isolate. Appl Environ Microbiol. 1981 Apr;41(4):849–856. doi: 10.1128/aem.41.4.849-856.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadin J. M., Stirland R. M., Preston N. W., Collard P. Identification of Vibrio cholerae by pyrolysis gas-liquid chromatography. Appl Microbiol. 1973 Jan;25(1):40–43. doi: 10.1128/am.25.1.40-43.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M. A., Guckert J. B., White D. C., Deck F. Effect of nutrient deprivation on lipid, carbohydrate, DNA, RNA, and protein levels in Vibrio cholerae. Appl Environ Microbiol. 1986 Oct;52(4):788–793. doi: 10.1128/aem.52.4.788-793.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp P., White R. W., Lander D. J. The hydrogenation of unsaturated fatty acids by five bacterial isolates from the sheep rumen, including a new species. J Gen Microbiol. 1975 Sep;90(1):100–114. doi: 10.1099/00221287-90-1-100. [DOI] [PubMed] [Google Scholar]
- Kepler C. R., Tove S. B. Biohydrogenation of unsaturated fatty acids. 3. Purification and properties of a linoleate delta-12-cis, delta-11-trans-isomerase from Butyrivibrio fibrisolvens. J Biol Chem. 1967 Dec 25;242(24):5686–5692. [PubMed] [Google Scholar]
- Kepler C. R., Tucker W. P., Tove S. B. Biohydrogenation of unsaturated fatty acids. V. Stereospecificity of proton addition and mechanism of action of linoleic acid delta 12-cis, delta 11-trans-isomerase from Butyrivibrio fibrisolvens. J Biol Chem. 1971 May 10;246(9):2765–2771. [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Initial phases of starvation and activity of bacteria at surfaces. Appl Environ Microbiol. 1983 Nov;46(5):978–984. doi: 10.1128/aem.46.5.978-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiw L. L., Boylen C. W., Tyson B. J. Lipid composition of growing and starving cells of Arthrobacter crystallopoietes. J Bacteriol. 1972 Jul;111(1):103–111. doi: 10.1128/jb.111.1.103-111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G., Morita R. Y. Starvation-Survival Physiological Studies of a Marine Pseudomonas sp. Appl Environ Microbiol. 1983 Apr;45(4):1206–1211. doi: 10.1128/aem.45.4.1206-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonell M. T., Hood M. A. Isolation and characterization of ultramicrobacteria from a gulf coast estuary. Appl Environ Microbiol. 1982 Mar;43(3):566–571. doi: 10.1128/aem.43.3.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makula R. A. Phospholipid composition of methane-utilizing bacteria. J Bacteriol. 1978 Jun;134(3):771–777. doi: 10.1128/jb.134.3.771-777.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinari L. A., Goldfine H., Panos C. Specificity of cyclopropane fatty acid synthesis in Escherichia coli. Utilization of isomers of monounsaturated fatty acids. Biochemistry. 1974 Apr 23;13(9):1978–1983. doi: 10.1021/bi00706a030. [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- Nichols P. D., Mayberry W. R., Antworth C. P., White D. C. Determination of monounsaturated double-bond position and geometry in the cellular fatty acids of the pathogenic bacterium Francisella tularensis. J Clin Microbiol. 1985 May;21(5):738–740. doi: 10.1128/jcm.21.5.738-740.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., Stringer W. F. Lipid Composition of a Psychrophilic Marine Vibrio sp. During Starvation-Induced Morphogenesis. Appl Environ Microbiol. 1984 Mar;47(3):461–466. doi: 10.1128/aem.47.3.461-466.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEURBRANDT G., BLOCH K. Unsaturated fatty acids in microorganisms. J Biol Chem. 1962 Jul;237:2064–2068. [PubMed] [Google Scholar]
- Schairer H. U., Overath P. Lipids containing trans-unsaturated fatty acids change the temperature characteristic of thiomethylgalactoside accumulation in Escherichia coli. J Mol Biol. 1969 Aug 28;44(1):209–214. doi: 10.1016/0022-2836(69)90416-1. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Degradation of cell constituents by starved Streptococcus lactis in relation to survival. J Gen Microbiol. 1969 Nov;58(3):347–362. doi: 10.1099/00221287-58-3-347. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Microcultural study of bacterial size changes and microcolony and ultramicrocolony formation by heterotrophic bacteria in seawater. Appl Environ Microbiol. 1981 Feb;41(2):518–527. doi: 10.1128/aem.41.2.518-527.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]



