Abstract
In several animals, male genitalia create insemination wounds in areas outside the genital orifice of females. I report that such traumatic insemination (TI) occurs in the Drosophila bipectinata complex (Diptera: Drosophilidae) and illustrate a previously unknown evolutionary pathway for this behaviour. Flash fixation of mating pairs revealed the dual function of the paired claw-like basal processes, previously misidentified as a bifid aedeagus: (i) penetration of the female body wall near the genital orifice and (ii) sperm transfer into the genital tract through the wounds. Basal processes in closely related species (Drosophila ananassae and Drosophila pallidosa) also wounded females but did not transfer sperm; this represents a transitional state to TI as observed in the bipectinata complex. Copulatory wounding is suggested to occur in other allied species of the Drosophila melanogaster species group, including D. melanogaster. Ubiquitous sexual conflicts over mating may have led to the evolution of novel intromittent organs for insemination.
Keywords: traumatic insemination, copulatory wounding, genital evolution, Drosophila
1. Introduction
In several animals, males transfer sperm through wounds made on the female's body, while the genital orifice is used in oviposition. Such traumatic insemination (TI; defined here as any mode of insemination achieved by trauma in a broad sense) is uncommon, but occurs in a wide range of taxa, including flatworms (Michiels & Newman 1998) and heteropterans (Carayon 1966; Siva-Jothy 2006; Tatarnic et al. 2006). Although this behaviour symbolizes possible conflicts over mating between the sexes, its evolutionary origin is not well understood.
Traumatic insemination is especially rare in taxa with separate male and female genders and is largely confined to the Cimicomorpha (bed bugs and allied heteropterans; Carayon 1966; Siva-Jothy 2006; Tatarnic et al. 2006). At least, three independent evolutions of TI have occurred in this group (Tatarnic et al. 2006). In two lineages (Cimicoidea and Miridae), TI is extragenitalic, with the male piercing the female's body wall outside the genital tract (Carayon 1966; Siva-Jothy 2006; Tatarnic et al. 2006). TI may have evolved in this group as a coercive mating strategy, providing males with a means to circumvent female pre- or post-mating resistance (Eberhard 1985; Stutt & Siva-Jothy 2001; Arnqvist & Rowe 2005).
Sperm transfer through the wound does not always accompany copulatory wounding. For example, male bean weevils Callosobruchus maculatus damage the female genital tract with their spiny intromittent organ during copulation, but no sperm is transferred through the wounds (Crudgington & Siva-Jothy 2000). However, we know nothing about the evolutionary relationship between copulatory wounding and TI. I report that copulatory wounding is common in the Drosophila melanogaster species group (genus Drosophila; subgenus Sophophora) and that this may have led to the evolution of a pair of novel functional intromittent organs used for TI.
2. Material and methods
I studied 21 species, representing 9 out of 12 species subgroups of the D. melanogaster species group, with special emphasis on the D. ananassae subgroup (figures 1b and 2; see also table 1 in the electronic supplementary material for details). I examined two strains in D. ananassae and D. pseudoananassae. Flies were maintained in glass vials (3 cm in diameter and 10.5 cm in height) containing cornmeal–malt medium at 20±1°C under a light : dark cycle of 14 : 10 h.
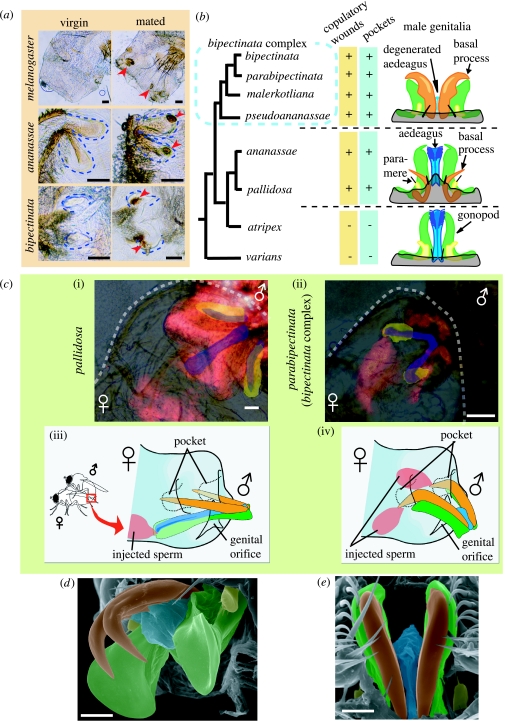
Figure 1.
(a) Comparison of virgin and mated female genitalia in three representative Drosophila species, with pockets outlined (dashed lines). Posterior to left. Mated females have melanized patches (arrowheads). (b) Copulatory wounds, pockets in female genitalia and the schematics of male genitalia of the ananassae subgroup (in part) with their proposed phylogeny. (c) Genitalic coupling of two representative species: (i,ii) top, laser scan micrographs with the female bodies outlined (dashed lines) and (iii,iv) bottom, schematics. In micrographs, ejaculates (and the male bodies) are stained with rhodamine-B (red). Pockets are highlighted in yellow, while the aedeagus and basal processes are in blue. In the schematics, male structures are drawn in different colours as in (b), and oviscapts and parameres are omitted. The schematics are not scaled. (d,e) Scanning electron micrograph of the male genitalia of D. pseudoananassae (d, postero-lateral view) and D. bipectinata (e, ventral view). Parts are highlighted in different colours following the schematics of (b) and (c). Scale bars, 50 μm in (a) and (c), 20 μm in (d,e).
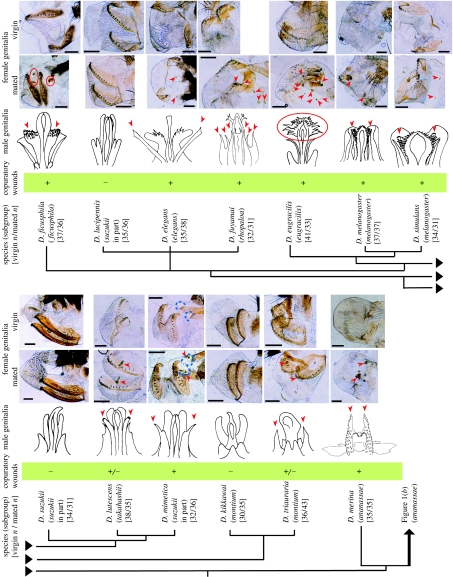
Figure 2.
Genitalia and copulatory wounds in the D. melanogaster species group. In light micrographs of female genitalia (posterior to left; scale bars, 100 μm), arrowheads or circles (red) mark melanized patches, indicating repair of wounds probably produced during copulation. Pocket-shaped structures were detected in female D. mimetica (outlined by the dashed lines). Hairy structures are omitted in the schematics of male genitalia. Arrowheads or circles mark the candidate wounding organs, estimated by the number, location, shape and size of melanized patches in females. Fixation of mating pairs verified the prediction in D. mimetica. The candidate wounding organs are not identical to the candidate sperm transfer organ, except in the bipectinata complex (figure 1), indicating that traumatic insemination is not likely to occur.
For each strain, I placed at least thirty 5-day-old females in a 1 : 1 sex ratio with males for 5 days (5–15 pairs per vial). I then dissected them in phosphate-buffered saline (PBS) and examined them under an Olympus BH2 light microscope. Virgin females of the same age (10 days old; n≥30 per strain) served as controls. Pilot studies revealed that, for the species used in this study, 5-day-old females achieve almost 100% insemination by the second day of pairing. An additional 3 days allowed for the development of detectable melanized patches, which are indicative of wound repair. I examined the morphology of the male phallic organs using light microscopy and a JEOL JSM-5310LV scanning electron microscope (SEM), both with and without pre-cleaning in KOH solution. The homology and terminology of male genitalia are not yet fully established for this group. I follow the terminology of Hu & Toda (2001).
I conducted the following experiment to visualize the genitalic coupling and insemination process into D. mimetica, D. ananassae, D. pallidosa and all four species of the D. bipectinata species complex (D. bipectinata, D. parabipectinata, D. malerkotliana and D. pseudoananassae). Virgins of both sexes (7–10 days old) were introduced in rearing vials (one to three pairs per vial). Males were fed Formula 4-24 Instant Drosophila Medium Blue (Carolina Biological Supply Company), prepared with 0.004 M rhodamine-B fluorescent dye solution (Wako Pure Chemical Industries), for 2 days before pairing. I flash froze the copulating pairs in liquid nitrogen 5 min after the initiation of copulation as described by Jagadeeshan & Singh (2006) and carefully removed and mounted the abdomens with 10% KOH and 2% SDS solution. I immediately observed and photographed the successful preparations (n≥5 per strain) under both a light microscope and a Zeiss LSM-410 laser scan microscope. Similar experiments without rhodamine-B treatment served as autofluorescence controls.
I constructed a phylogeny for the study species (figures 1b and 2) based on the recent molecular phylogenetic studies of Kopp & Barmina (2005) for relationships among the subgroups, and Kopp (2006) for relationships within the bipectinata complex. I consulted Schawaroch (2002) and Da Lage et al. (2007) for species not sampled in the other studies.
3. Results and discussion
All mated, but no virgin, females of 14 species (representing seven subgroups) had melanized patches on their genital regions, indicating wound repair (Pathak 1993; figures 1a and 2). In two species, melanized patches were not always present (figure 2). The number, size, shape and location of melanized patches differed among species (figures 1a and 2), but in five species no signs of wounding were detected.
In the species of the bipectinata complex, D. ananassae and D. pallidosa (the ananassae subgroup), melanized patches at the bottom of paired blind invaginations (‘pockets’) near the genital orifice (figure 1a,c) enabled in situ identification of the specific organ of male genitalia which carries out the wounding (similar pockets were also found in D. mimetica (suzukii subgroup); figure 2). The phallic organ (central part of male genitalia) of D. ananassae and D. pallidosa consists of the aedeagus, gonopods, basal processes and parameres (figure 1b). Flash fixation of copulating pairs of these species revealed that the lanceolate basal processes stabbed the pockets, and fluorescent-marked semen was ejaculated via the aedeagus into the female genital orifice figure 1b,c(i,iii).
In the bipectinata complex, the basal processes are well developed as twin claw-like structures (figure 1b,d,e). Using SEM, I detected a degenerate, transparent, tube-like true aedeagus between the bases of the basal processes (figure 1d,e), suggesting their previous identification as bifid aedeagus (Book & Wheeler 1972; Eberhard & Ramirez 2004) is incorrect. TI clearly occurs in the bipectinata complex, as the basal processes pierce the pockets during copulation and sperm is ejaculated through the wounds but not through the genital orifice (figure 1c(ii,iv) for unique laterality in D. pseudoananassae, see figure 1 in the electronic supplementary material). The basal processes of this group have a groove on the dorsal surface which may transport semen. Evolutionarily, the insemination function may have been transferred from the aedeagus to the neighbouring basal processes in the common ancestor of the bipectinata complex (figure 1b,d,e), resulting in a separation of the sperm inlet (pockets) and the egg outlet (genital orifice).
While the D. melanogaster species group consists of many more species (200), my results show clearly that copulatory wounding occurs in several lineages implying that it has evolved several times independently (figures 1 and 2). In all lineages, the sperm-receiving pockets are simple invaginations of the body wall, and ejaculates are directly injected into the female genital tract but not into the haemocoel (no intraperitoneal insemination). This contrasts with the Cimicomorpha where females show various types of sperm-receiving organs (paragenitalia or spermalege; Carayon 1966; Siva-Jothy 2006; Tatarnic et al. 2006). In bed bugs, this unique organ ameliorates the costs of mating associated with TI (Morrow & Arnqvist 2003; Reinhardt et al. 2003), while the benefits for males and the costs for females incurred by copulatory wounding are presently unknown for Drosophila. However, TI may facilitate the transfer of seminal fluid proteins to the female which in D. melanogaster reduce female fitness while enhancing male success in sperm competition (e.g. Chapman 2001). As such, copulatory wounding may be beneficial for males, or the resultant wounds may deter females from subsequently mating with rivals. In either case, the coupling of insemination and wounding (equal to TI) may be an excellent male solution to possible conflicts over mating, because females cannot develop a complete avoidance mechanism to wounding, such as deeper pockets, without risking to become infertile. Future studies should take into account these and other effects of physical damage when studying the reproductive biology of this model organism.
Acknowledgments
I thank M. T. Kimura for assistance in fly rearing, M. J. Toda for help with genitalic terminology, M. T. Kimura, M. Kondoh, K. Akutsu, T. Ide, M. Matsuda and the Tucson Drosophila Species Stock Centre for their fly strains, and M. T. Kimura, F. Hayashi and two anonymous referees for their comments on the manuscript. This work was supported by a Grant-in-Aid for Scientific Research (nos. 16770017 and 19770046) from the Japan Ministry of Education, Culture, Sports, Science and Technology.
Supplementary Material
Female genitalia and insemination in Drosophila pseudoananassae
List of Drosophila strains examined
References
- Arnqvist G, Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Book I.R, Wheeler M.R. The Drosophila melanogaster species group. Univ. Texas Publ. 1972;7213:1–102. [Google Scholar]
- Carayon J. Traumatic insemination and the paragenital system. In: Usinger R.L, editor. Monograph of the Cimicidae (Hemiptera-Heteroptera) Entomological Society of America; College Park, MD: 1966. pp. 81–166. [Google Scholar]
- Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. doi:10.1046/j.1365-2540.2001.00961.x [DOI] [PubMed] [Google Scholar]
- Crudgington H.S, Siva-Jothy M.T. Genital damage, kicking and early death. Nature. 2000;407:855–856. doi: 10.1038/35038154. doi:10.1038/35038154 [DOI] [PubMed] [Google Scholar]
- Da Lage J.-L, Kergoat G.J, Maczkowiak F, Silvain J.-F, Cariou M.-L, Lachaise D. A phylogeny of Drosophilidae using the Amyrel gene: questioning the Drosophila melanogaster species group boundaries. J. Zool. Syst. Evol. Res. 2007;45:47–63. doi:10.1111/j.1439-0469.2006.00389.x [Google Scholar]
- Eberhard W.G. Harvard University Press; Cambridge, MA: 1985. Sexual selection and animal genitalia. [Google Scholar]
- Eberhard W.G, Ramirez N. Functional morphology of the male genitalia of four species of Drosophila: failure to confirm both lock and key and male–female conflict predictions. Ann. Entomol. Soc. Am. 2004;97:1007–1017. doi:10.1603/0013-8746(2004)097[1007:FMOTMG]2.0.CO;2 [Google Scholar]
- Hu Y.-G, Toda M.J. Polyphyly of Lordiphosa and its relationships in Drosophilinae (Diptera: Drosophilidae) Syst. Entomol. 2001;26:15–31. doi:10.1046/j.1365-3113.2001.00135.x [Google Scholar]
- Jagadeeshan S, Singh R.S. A time-sequence functional analysis of mating behaviour and genital coupling in Drosophila: role of cryptic female choice and male sex-drive in the evolution of male genitalia. J. Evol. Biol. 2006;19:1058–1070. doi: 10.1111/j.1420-9101.2006.01099.x. doi:10.1111/j.1420-9101.2006.01099.x [DOI] [PubMed] [Google Scholar]
- Kopp A. Basal relationships in the Drosophila melanogaster species group. Mol. Phylogenet. Evol. 2006;39:787–798. doi: 10.1016/j.ympev.2006.01.029. doi:10.1016/j.ympev.2006.01.029 [DOI] [PubMed] [Google Scholar]
- Kopp A, Barmina O. Evolutionary history of the Drosophila bipectinata species complex. Genet. Res. Camb. 2005;85:23–46. doi: 10.1017/s0016672305007317. doi:10.1071/S0016672305007317 [DOI] [PubMed] [Google Scholar]
- Michiels N.K, Newman L.J. Sex and violence in hermaphrodites. Nature. 1998;391:647. doi:10.1038/35527 [Google Scholar]
- Morrow E.H, Arnqvist G. Costly traumatic insemination and a female counteradaptation in bed bugs. Proc. R. Soc. B. 2003;270:2377–2381. doi: 10.1098/rspb.2003.2514. doi:10.1098/rspb.2003.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak J.P.N. Cell-mediated defence reactions in insects. In: Pathak J.P.N, editor. Insect immunity. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1993. pp. 47–58. [Google Scholar]
- Reinhardt K, Naylor R, Siva-Jothy M.T. Reducing a cost of traumatic insemination: female bed bugs evolve a unique organ. Proc. R. Soc. B. 2003;270:2371–2375. doi: 10.1098/rspb.2003.2515. doi:10.1098/rspb.2003.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schawaroch V. Phylogeny of a paradigm lineage: the Drosophila melanogaster species group (Diptera: Drosophilidae) Biol. J. Linn. Soc. 2002;76:21–37. doi:10.1046/j.1095-8312.2002.00044.x [Google Scholar]
- Siva-Jothy M.T. Trauma, diseases and collateral damage: conflict in cimicids. Phil. Trans. R. Soc. B. 2006;361:269–275. doi: 10.1098/rstb.2005.1789. doi:10.1098/rstb.2005.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutt A.D, Siva-Jothy M.T. Traumatic insemination and sexual conflict in the bed bug Cimex lectularius. Proc. Natl Acad. Sci. USA. 2001;98:5683–5687. doi: 10.1073/pnas.101440698. doi:10.1073/pnas.101440698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarnic N.J, Cassis G, Hochuli D.F. Traumatic insemination in the plant bug genus Coridromius Signoret (Heteroptera: Miridae) Biol. Lett. 2006;2:58–61. doi: 10.1098/rsbl.2005.0394. doi:10.1098/rsbl.2005.0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Female genitalia and insemination in Drosophila pseudoananassae
List of Drosophila strains examined