Abstract
Cormorants hunt both benthic (sedentary) and pelagic (motile) prey but it is not known if the energy costs of foraging on these prey differ. We used respirometry to measure the costs of diving in double-crested cormorants (Phalacrocorax auritus) foraging either for sedentary (fish pieces) or motile (juvenile salmon) prey in a deep dive tank. Short dives for sedentary prey were more expensive than dives of similar duration for motile prey (e.g. 20% higher for a 10 s dive) whereas the reverse was true for long dives (i.e. long dives for motile prey were more expensive than for sedentary prey). Across dives of all durations, the foraging phase of the dive was more expensive when the birds hunted motile prey, presumably due to pursuit costs. The period of descent in all the dives undertaken appears to have been more expensive when the birds foraged on sedentary prey, probably due to a higher swimming speed during this period.
Keywords: cormorant, diving, energetics, foraging, prey, respirometry
1. Introduction
Cormorants show unusually diverse foraging behaviours for a diving bird, taking both pelagic and benthic prey during a foraging trip (Grémillet et al. 1999). In environments offering a variety of foraging choices, optimal foraging theory predicts that predators such as cormorants will adjust their hunting strategy within their physiological limits to achieve maximum net rate of energy gain (Krebs & Kacelnik 1991; Grémillet et al. 1999). The most notable physiological constraints in cormorants are their limited visual acuity (Strod et al. 2004) and high rates of energy expenditure due to heat loss in cold water (Enstipp et al. 2006). However, the differing energy costs of preying on sedentary and motile prey have not been investigated.
Since pursuit involves changes in speed which affect power requirements (Schmid et al. 1995), it can be hypothesized that the cost of foraging on motile prey is higher than foraging on sedentary prey. However, no measurements have been made to test this. As an estimate of foraging cost, we measured the rate of oxygen uptake by double-crested cormorants (Phalacrocorax auritus) between foraging dives. The cormorants foraged either on ‘sedentary prey’ (pieces of chopped Pacific herring, Clupea pallasi, on the tank bottom) or ‘motile prey’ in the water column (live, juvenile coho salmon, Oncorhynchus kisutch).
2. Material and methods
Five adult double-crested cormorants (mass range: 1.77–2.23 kg) were used. They were housed communally in sheltered outdoor pens (8×4×5 m high) with water tank access, at the University of British Columbia (UBC). Details of bird care are given in Enstipp et al. (2006).
(a) Dive tank and training protocol
The dive tank (12×5 m diameter) was covered with flexible mesh except for a wooden base in one-quarter that held a truncated, clear acrylic pyramid. This served as a respirometry chamber (65 l). The tank was filled with de-chlorinated, fresh water up to 9.5 m depth. The birds were trained to dive singly from the respirometry chamber to the bottom of the tank (Enstipp et al. 2006) or to a netting floor at 5 m depth. Water temperature ranged between 13.8 and 17.3°C.
(b) Experiments
A bird was placed on the water inside the respirometer. Each bird undertook both ‘sedentary prey’ and ‘motile prey’ experiments.
During sedentary prey experiments, pieces of herring (15–30 g) were scattered randomly at the bottom of the dive tank or on the netting floor. For motile prey experiments, netting was not used. Approximately 300 juvenile coho salmon (5–8 cm, 1.4–4.8 g) were obtained from Inch Creek Hatchery (Mission, BC) and maintained in de-chlorinated, aerated tanks at UBC. Approximately 50 fish were present in the tank before the start of each trial. Fish roamed freely throughout the water column, either individually or as part of a school. Thus the depth to which the cormorant dived each time varied considerably during motile prey experiments.
Viewing the chamber discretely from a hut, we were able to record the start and end of each dive. A monitor within the hut received simultaneous pictures from four underwater cameras (Lorex, Mbrands, Scarborough, Canada), enabling maximum dive depth and number of prey items taken per dive to be recorded.
(c) Respirometry
Gas exchange was measured using standard, negative pressure, open-flow respirometry. Air was drawn, at 90–100 l min−1, through a series of small holes in the respirometry chamber, a 0.5 l condensation trap and a mass flowmeter (Sierra Instruments, Inc., Monterrey, USA), using a vacuum pump (Piston pump, Gast Manufacturing, Inc., Benton Harbour, USA). This high rate ensured thorough mixing within the chamber and minimized the residual time constant of the system (3 s). A subsample of air was drawn downstream of the flowmeter, through a column containing indicating drierite, and then through O2 and CO2 analysers (ML206, ADInstruments) at a flow rate of 250 ml min−1. The respirometry system was tested for leaks with nitrogen injections. The voltage outputs of the gas analyser and flowmeter were recorded at a sampling frequency of 100 Hz (downsampled to 4 Hz for analysis) by a ML750 A/D converter (ADInstruments, Bella Vista, Australia) and Chart software. Analyser drift and lag time of the respirometry system were corrected during data analysis.
The volume of oxygen uptake (VO2, ml) after a dive was calculated using modifications of the instantaneous equation developed by Woakes & Butler (1983) to enable measurements of changes in oxygen uptake over short time periods (2 s). VO2 over the period at the surface after a dive (the surface period) was assumed to represent the quantity of oxygen consumed during the surface period and during the dive (Halsey et al. 2005), i.e. the energetic cost of the dive cycle.
(d) Statistical analyses
At the beginning of our experiments, birds would sometimes submerge just below the surface cover to swim in a circle, with no apparent interest in foraging. These dives were excluded from the analysis, as were dives when the surface period exceeded 45 s. Not all dives included in analyses involved prey capture.
Statistics were conducted using JMP v. 5.1.2 (SAS Institute, USA). An ANCOVA model was tested with VO2 over the surface period regressed against dive duration. This analysis included prey type as a categorical variable, individual bird as a random factor, several covariates (maximum dive depth, duration of the surface period and bird mass) and second-order interactions. Non-significant interactions were removed by stepwise backwards elimination. Parameter estimates from the resulting model were used to plot and regress VO2 against dive duration independently for both prey types, where VO2 was adjusted to control for dive depth, duration of the surface period and body mass (adjusted VO2). The Johnson–Neyman technique was then used to identify regions where the two regressions were significantly different (White 2003).
3. Results
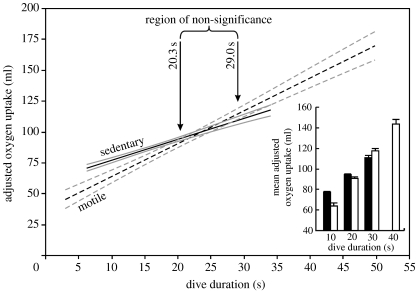
The analyses included 890 dives (table 1). There was a positive relationship between VO2 during the surface period and dive duration (p<0.001), and a significant effect of prey type (p<0.01). There was a significant interaction between prey type and dive duration (p<0.001). The slope of the relationship between adjusted VO2 and dive duration is steeper for dives for motile prey (figure 1). The elevations of the two regression slopes of adjusted VO2 against dive duration were significantly different for dives shorter than 20.3 s (sedentary>motile) and longer than 29.0 s (motile>sedentary; figure 1). For example, dives of 10 s duration for sedentary prey are 20% more expensive than dives for motile prey (figure 1, inset). Dives of 20 s duration for sedentary prey are 4% more expensive than dives for motile prey. Most (72%) dives for sedentary prey are shorter than 20 s, as are 54% of dives for motile prey. Only 2 and 18% of dives for sedentary and motile prey, respectively, were longer than 29 s.
Table 1.
Dive durations and subsequent surface period durations of five cormorants diving for sedentary and motile prey.
| prey type | sedentary | motile |
| n | 594 | 296 |
| dive duration | ||
| mean±s.e.m. | 16.9±0.2 | 20.6±0.5 |
| range | (6.3–34.0) | (3–49.8) |
| duration of surface-period | ||
| mean±s.e.m. | 13.3±0.3 | 13.7±0.5 |
| range | (2.3–71.5) | (0.5–127.0) |
Figure 1.
The relationship between adjusted oxygen uptake and dive duration in five cormorants foraging on either sedentary or motile prey. Oxygen uptake is adjusted to mean duration of the surface period (13.4 s), mean dive depth (5.6 m) and mean bird mass (2.1 kg). Regressions: sedentary prey, solid, =1.68x+60.4, R2=0.23; motile prey, dashed, =2.66x+37.6, R2=0.38. Grey lines indicate 95% confidence limits. The region of non-significance (see text) is the range of dive durations where the elevations of the two regressions are not significantly different. Inset: mean adjusted oxygen uptake+s.e.m. for dives for sedentary prey (filled bars) of 10, 20 and 30 s and for dives for motile prey (open bars) of 10, 20, 30 and 40 s.
4. Discussion
Cormorants prey upon a range of species that vary in nutritional and energetic value (Brugger 1993; Grémillet et al. 2003) and this should affect foraging preferences (Grémillet et al. 1999). We have shown that in cormorants there is also a difference in the energetic costs of diving for sedentary and motile prey, which may also be an important factor in prey choice. The prey type associated with more costly dives depends upon dive duration.
Assuming that the cost of descent from and ascent to the respirometry chamber is unrelated to dive duration (with dive depth controlled for), the regression slope represents the rate of oxygen consumption during the foraging phase of the dive (Halsey et al. 2005). This indicates that the rate of oxygen consumption during the foraging phase is higher for dives for motile prey (figure 1). Furthermore, the value of the regression lines at the shortest dive durations, i.e. when the duration of the foraging phase was close to 0 s, indicates the energy costs of descent and ascent, plus the energy cost during the adjusted surface period. This value is higher for dives for sedentary prey, suggesting that the energy costs of descent and ascent during these dives are higher.
Once in the foraging phase of the dive, cormorants expend more energy capturing motile, coho salmon than when taking fish pieces. The most likely reason for this is the increased costs of a higher swimming speed in the pursuit of motile prey (Schmid et al. 1995). The fact that the energy costs of the travel periods within a dive were higher, when the cormorants foraged on fish pieces, may be explained by the greater initial acceleration and faster rate of descent observed in the cormorants during these dives. This is presumably because after a few sedentary trials, they learnt that food was located on the tank floor and so headed straight for the bottom upon submergence, descending rapidly, perhaps to increase their chances of capturing the food before it disappeared, e.g. was eaten by a conspecific. In contrast, when searching for coho salmon, the cormorants descended more slowly, presumably searching for the fish.
In terms of the energetic costs of entire dives, foraging for sedentary prey was energetically more costly than foraging for motile prey up to dive durations of 20.3 s. Beyond 20 s, virtually all dives for sedentary prey were of similar cost to dives for motile prey (figure 1). We suggest that short dives for sedentary items in our study were probably more costly because the descent phase, which was particularly expensive when foraging on sedentary prey, made up a large proportion of the dive. In long dives for sedentary prey, the descent phase was a smaller proportion and the bottom phase, which was cheaper than in dives for motile prey, made up a large proportion of the dive, making these dives cheaper than those of equivalent length for motile prey.
The depths and durations of dives by double-crested cormorants in the wild are similar to those of the birds in the present study (Johnsgard 1993). The variations in diving behaviour in response to prey type presented here, and the associated differences in energetics, may well be representative of cormorants diving in the wild. Data logging technology would serve to elucidate this issue (Grémillet et al. 1999) with regards to its impact on their ecology. Though such data for wild double-crested cormorants are not presently available, it appears that for cormorants in general, high-speed prey pursuits are not particularly common (e.g. Ropert-Coudert et al. 2006). This may be not only due to the difficulty in visually identifying prey from a distance but also due to the high-energetic costs of such pursuits.
Acknowledgments
Experimental procedures were approved by the UBC Animal Care Committee and in compliance with the Canadian Council on Animal Care.
This research was supported by NSERC, NERC and The Company of Biologists. Inch Creek Hatchery generously donated coho salmon. David Grémillet kindly provided equipment.
References
- Brugger K. Digestibility of three fish species by double-crested cormorants. Condor. 1993;95:25–32. doi:10.2307/1369383 [Google Scholar]
- Enstipp M.R, Grémillet D, Jones D. The effect of depth, temperature and food ingestion on the foraging energetics of a diving endotherm, the double-crested cormorant (Phalcrocorax auritus) J. Exp. Biol. 2006;209:845–859. doi: 10.1242/jeb.02064. doi:10.1242/jeb.02064 [DOI] [PubMed] [Google Scholar]
- Grémillet D, Wilson R.P, Storch S, Yann G. Three-dimensional space utilisation by a marine predator. Mar. Ecol. Prog. Ser. 1999;183:263–273. [Google Scholar]
- Grémillet D, Wright G, Lauder A, Carss D.N, Wanless S. Modelling the daily food requirements of wintering great cormorants: a bioenergetics tool for wildlife management. J. Appl. Ecol. 2003;40:266–277. [Google Scholar]
- Halsey L.G, Butler P.J, Woakes A.J. Breathing hypoxic gas affects the physiology as well as the diving behaviour of tufted ducks. Physiol. Biochem. Zool. 2005;78:273–284. doi: 10.1086/427053. doi:10.1086/427053 [DOI] [PubMed] [Google Scholar]
- Johnsgard P.A. Smithsonian Institution Press; Washington, DC: 1993. Cormorants, darters, and pelicans of the world. [Google Scholar]
- Krebs J.R, Kacelnik A. Decision making. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. Blackwell; Oxford, UK: 1991. pp. 105–137. [Google Scholar]
- Ropert-Coudert Y, Grémillet D, Kato A. Swim speeds of free-ranging great cormorants. Mar. Biol. 2006;149:415–422. doi:10.1007/s00227-005-0242-8 [Google Scholar]
- Schmid D, Grémillet D.J.H, Culik B.M. Energetics of underwater swimming in the great cormorant (Phalacrocorax carbo sinsensis) Mar. Biol. 1995;123:875–881. doi:10.1007/BF00349133 [Google Scholar]
- Strod T, Arad Z, Izhaki I, Katzir G. Cormorants keep their power: visual resolution in a pursuit-diving bird under amphibious and turbid conditions. Curr. Biol. 2004;14:R376–R377. doi: 10.1016/j.cub.2004.05.009. doi:10.1016/j.cub.2004.05.009 [DOI] [PubMed] [Google Scholar]
- White C.R. Allometric analysis beyond heterogenous regression slopes: use of the Johnson–Neyman technique in comparative biology. Physiol. Biochem. Zool. 2003;76:135–140. doi: 10.1086/367939. doi:10.1086/367939 [DOI] [PubMed] [Google Scholar]
- Woakes A.J, Butler P.J. Swimming and diving in tufted ducks, (Aythya fuligula), with particular reference to heart rate and gas exchange. J. Exp. Biol. 1983;107:311–329. [Google Scholar]