Abstract
Global climate change is driving rapid distribution shifts in marine ecosystems; these are well established for lower trophic levels, but are harder to quantify for migratory top predators. By analysing a 25-year sightings-based dataset, we found evidence for rapid northwards range expansion of the critically endangered Balearic shearwater Puffinus mauretanicus in northeast Atlantic waters. A 0.6°C sea surface temperature increase in the mid-1990s is interpreted as an underlying controlling factor, while simultaneous northward shifts of plankton and prey fish species suggests a strong bottom-up control. Our results have important conservation implications and provide new evidence for climate-driven regime shift in Atlantic ecosystems.
Keywords: climate change, trophic cascade, Atlantic Ocean, Balearic shearwater
1. Introduction
Global climate change is impacting both marine and terrestrial ecosystems (Reid et al. 1998; Thomas & Lennon 1999), with many species currently shifting their range polewards (Parmesan & Yohe 2003). In the northeast Atlantic, rising sea temperature is thought to be the major control behind northwards distribution shift at a variety of trophic levels, from phytoplankton (Reid et al. 1998; Beaugrand & Reid 2003; Richardson & Schoeman 2004) through to zooplankton (Beaugrand et al. 2002; Beaugrand & Reid 2003) and fish (Stebbing et al. 2002; Beare et al. 2004a,b; Genner et al. 2004; Perry et al. 2005). It has also been predicted that top predators at higher trophic levels will inevitably have to adapt to changing spatial distribution of their prey (Richardson & Schoeman 2004; McMahon & Hays 2006). However, long-term datasets quantifying large-scale climate-induced distribution changes in migratory marine top predators are lacking for the Atlantic region.
In this study, we use a 25-year sightings-based dataset to show that the post-breeding distribution of a critically endangered migratory seabird, the Balearic shearwater Puffinus mauretanicus, has rapidly expanded northwards in the last decade. We then discuss the controls on this range expansion, including changes in northeast Atlantic sea surface temperature (SST) and prey fish distribution. The Balearic shearwater has been selected for detailed study as (i) it is a declining species threatened with virtual extinction by 2050 (Oro et al. 2004; IUCN 2006), so understanding how external factors such as climate change affect adult survival is an urgent conservation requirement, and (ii) it is a well-recorded and mobile migratory seabird with a preference for coastal waters, and is therefore a useful and accessible ecological indicator of climate change. Previous research has shown that post-breeding concentrations of Balearic shearwaters along the French Biscay coast decreased markedly between 1982–1984 and 1990–2000 (Yésou 2003); our aim is to determine whether this decrease represents a change in distribution or simply reflects overall population decline.
2. Material and methods
Land-based sightings data for the Balearic shearwater, covering the period 1980–2003 (the last year for which complete data are available), were obtained from nine countries along the northwest European coastline (north of 48° N; figure 1). In a majority of cases, individual records have been peer-reviewed before acceptance by the relevant national or local ornithological records committee. Annual totals were compiled for each country or region; duplicate records were removed where timings suggest a single bird passed multiple watch-points.
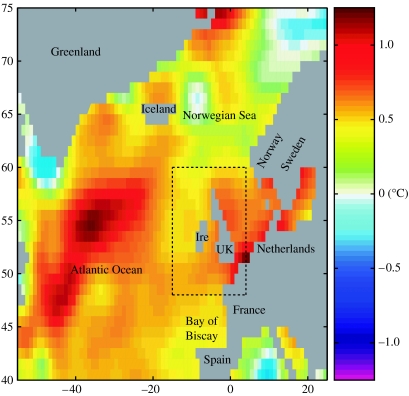
Figure 1.
Map of the study area, showing differences in annual mean SST (°C) between the two periods 1995–2005 and 1980–1994. Image resolution limited by climatological grid cell size. Dashed rectangle shows the UK and Ireland region used to calculate SST statistics in this study, where temperatures have risen by approximately 0.6°C since the mid-1990s.
Data used in this study are potentially affected by observer bias to varying degrees, as a result of (i) differences in observer effort, identification skills and optical aids, (ii) greater awareness of Balearic shearwaters due to taxonomic changes, and (iii) variable viewing conditions. Such factors are hard to quantify, and bias reduction is therefore difficult. However, as discussed below, spatial and temporal consistency of results is high, indicating that observer bias has not significantly affected the dataset.
3. Results
The most comprehensive sightings data for the period 1980–2003 are available from the UK and Ireland. In this region, relatively small numbers of Balearic shearwaters were recorded between 1980 and 1990, with annual totals of less than 500 birds per year. Numbers then rapidly increased through the mid-1990s and peaked at 3500 birds in 2001 (figure 2a). The largest numbers of birds (approx. 70%) are typically seen from southwest England, with smaller numbers penetrating north to Scotland. There is evidence for progressive northwards range expansion, with annual totals in southern England peaking in 2001, and those from Wales and Scotland peaking in 2003 (figure 2b). The change in status in UK waters is supported by long-term sightings data from manned bird observatories, for example, Portland Bird Observatory (figure 2b), where Balearic shearwaters have been recognized and recorded since the 1950s (Ash & Rooke 1954).
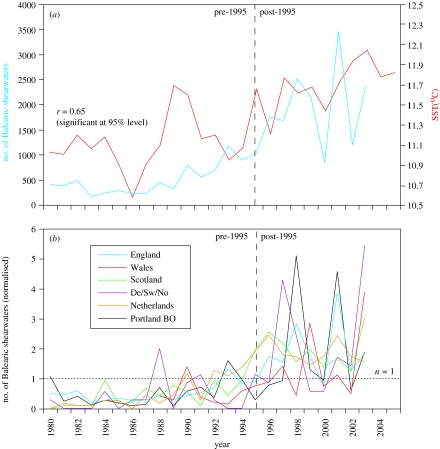
Figure 2.
(a) Time series for the period 1980–2003 showing annual totals of Balearic shearwaters in UK and Irish waters (blue), and annually averaged SST in the UK and Ireland region shown in figure 1 (red). (b) Time series showing numbers of Balearic shearwaters in northwest European waters from 1980 to 2003; note the consistent increase in numbers in the mid-1990s (vertical dashed line). Individual annual totals have been normalized relative to 1980–2003 average values; a value of 1.0 (represented by dotted line) is therefore equal to the long-term average, a value of 0.5 is half the long-term average, and a value of 2.0 is double the long-term average. Values for Denmark (De), Sweden (Sw) and Norway (No) have been combined. Portland BO, Portland Bird Observatory.
The rapid and sustained increase in numbers observed in UK waters since the mid-1990s has been repeated across northwest Europe (figure 2b), with additional evidence for a progressive northwards shift. Annual totals in northern France (Yésou 2003), England and The Netherlands all peaked between 1996 and 2001, whereas annual totals in countries north of 55° N (e.g. Scotland, Denmark, Sweden, Norway) all peaked in 2003, although the numbers involved in the latter region are relatively small. The temporal (decadal and inter-annual) and spatial (intra- and international) consistency of data from across northwest Europe (figure 2b), including those from long-term observatories, provides firm evidence for genuine range expansion and indicates that observer bias has not significantly affected the results.
4. Discussion
Rapid increases in northeast Atlantic SST have been observed in recent decades as part of larger-scale global warming of the ocean surface (Rayner et al. 2006). In order to assess potential impacts on Balearic shearwater distribution, we quantified the rise in SST for a region centred on the UK and Ireland (figures 1 and 2a) using the ship observation-based NOC1.1 dataset (Josey et al. 1999; dataset available at http://www.noc.soton.ac.uk/ooc/CLIMATOLOGY/index.php). Regionally averaged SST has increased throughout the period considered, with an upward shift in the mid-1990s that parallels the rise in Balearic shearwater numbers in UK and Irish waters. The mean SST values for 1980–1994 and 1995–2005 are 11.1±0.1 and 11.7±0.1°C, respectively, i.e. the surface temperature over the latter period was on average approximately 0.6°C warmer than the preceding 15 years (consistent with Rayner et al. 2006, their fig. 14d). Over the common data period (1980–2003), the correlation coefficient between numbers of Balearic shearwaters and regionally averaged SST is r=0.65; this value is significant at the 95% level (threshold for significance, rTh=0.40, note autocorrelation within the time series may increase this value). Our result thus suggests that Balearic shearwater numbers are linked to SST variability, although it does not establish a direct causal connection.
To gain insights into the mechanisms by which rising SST may be driving the observed change in Balearic shearwater distribution, we must investigate spatial and temporal changes in prey species. The dominant prey of the Balearic shearwater is small pelagic fish, particularly anchovy Engraulis encrasicholus and sardine Sardina pilchardus (Arcos & Oro 2002; Yésou 2003). Along the French Biscay coast, specifically in areas where Balearic shearwaters used to occur in greatest numbers, these two fish species have decreased dramatically since the mid- to late-1990s (Poulard & Blanchard 2005; ICES 2006); coincident with a local decrease in Balearic shearwater numbers (Yésou 2003) and increased northwards dispersal (figure 2). Further north, fisheries data provide indications for a rapid increase in both anchovy and sardine abundance in UK waters during the mid-1990s (Armstrong et al. 1999; Beare et al. 2004b), while several other fish species with southern biogeographic affinities show an apparent northwards distribution shift in the mid-1990s that is strongly correlated with rising SST (Stebbing et al. 2002; Beare et al. 2004a; Genner et al. 2004; Perry et al. 2005; Poulard & Blanchard 2005).
There is also increasing evidence for climate-related distribution shifts in the plankton that directly, or indirectly, provide food for prey fish species. Copepod species associated with warm temperate waters have moved north by 10° latitude in northeast Atlantic waters since the early to mid-1980s (Beaugrand et al. 2002; Beaugrand & Reid 2003), with a simultaneous retreat of subarctic and arctic species. These shifts are strongly correlated with increasing SST. At the very base of the food chain, proxies for local phytoplankton abundance demonstrate a simultaneous stepwise change (Reid et al. 1998; Beaugrand & Reid 2003). Increasing SST therefore appears to initiate a cascade effect, with subsequent changes in phytoplankton occurrence affecting their herbivore grazers and ultimately their predators in a bottom-up manner (Richardson & Schoeman 2004; Frederiksen et al. 2006). Our work provides the first evidence for this cascade affecting the large-scale distribution of a migratory top predator in Atlantic waters, although Veit et al. (1996) have previously documented a similar relationship in the northeast Pacific Ocean.
It is important to identify major potential threats to the survival of endangered species before their influence becomes irreversible, so that effective conservation strategy can be planned and implemented (McMahon & Hays 2006). The breeding population of the Balearic shearwater is undergoing a serious, and potentially terminal, decline (Oro et al. 2004), and the breeding distribution remains restricted to the Balearic Islands. However, our results indicate a rapid northwards expansion in post-breeding distribution since the mid-1990s, while a simultaneous decrease in numbers using northern Biscay may point towards a genuine distribution shift (Yésou 2003). We propose that this phenomenon has occurred in response to climate-driven shifts in prey distribution. Increased dispersal range and/or decreased foraging success at sea may be contributing to the species unusually low adult survival rate (Oro et al. 2004). Early indications for northwards range expansion of other scarce migratory seabirds (e.g. Fea's Petrel (Pterodroma feae); Steele 2006), suggest that they could also be affected in a similar fashion. Further research should therefore be directed towards predicting future distribution changes in response to predicted SST increases, in combination with ongoing (pan-European) long-term monitoring initiatives.
Acknowledgments
We would like to thank all observers who collected and contributed Balearic Shearwater data for this study. We are grateful to Jacques Massé, Marie-Joëlle Rochet and Patrick Le Mao from IFREMER for advice on Bay of Biscay fisheries data. The referees are thanked for constructive reviews that improved the manuscript.
Supplementary Material
References
- Arcos J.M, Oro D. Significance of fisheries discards for a threatened Mediterranean seabird, the Balearic shearwater Puffinus mauretanicus. Mar. Ecol. Prog. Ser. 2002;139:209–220. [Google Scholar]
- Armstrong M.J, Dickey-Collas M, McAliskey M, McCurdy W.J, Burns C.A, Peel J.A.D. The distribution of anchovy Eugralis encrasicolus in the northern Irish Sea from 1991 to 1999. J. Mar. Biol. Assoc. UK. 1999;79:955–956. doi:10.1017/S0025315499001162 [Google Scholar]
- Ash J.S, Rooke K.B. Balearic shearwaters off the Dorset coast. Br. Birds. 1954;47:285–296. [Google Scholar]
- Beare D.J, Burns F, Greig A, Jones E.G, Peach K, Kienzle M, Mckenzie E, Reid D.G. Long-term increases in prevalence of North Sea fishes having southern biogeographic affinities. Mar. Ecol. Prog. Ser. 2004a;284:269–278. [Google Scholar]
- Beare D.J, Burns F, Jones E, Peach K, Portilla E, Greig T, McKenzie E, Reid D. An increase in the abundance of anchovies and sardines in the north-western North Sea since 1995. Global Change Biol. 2004b;10:1209–1213. doi:10.1111/j.1529-8817.2003.00790.x [Google Scholar]
- Beaugrand G, Reid P.C. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Global Change Biol. 2003;9:801–817. doi:10.1046/j.1365-2486.2003.00632.x [Google Scholar]
- Beaugrand G, Reid P.C, Ibañez F, Lindley J.A, Edwards M. Reorganization of North Atlantic marine copepod biodiversity and climate. Science. 2002;296:1692–1694. doi: 10.1126/science.1071329. doi:10.1126/science.1071329 [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Edwards M, Richardson A.J, Halliday N.C, Wanless S. From plankton to top predators: bottom-up control of a marine food web across four trophic levels. J. Anim. Ecol. 2006;75:1259–1268. doi: 10.1111/j.1365-2656.2006.01148.x. doi:10.1111/j.1365-2656.2006.01148.x [DOI] [PubMed] [Google Scholar]
- Genner M.J, Sims D.W, Wearmouth V.J, Southall E.J, Southward A.J, Henderson P.A, Hawkins S.J. Regional climatic warming drives long-term community changes of British marine fish. Proc. R. Soc. B. 2004;271:655–661. doi: 10.1098/rspb.2003.2651. doi:10.1098/rspb.2003.2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICES 2006 Report of the working group on acoustic and egg surveys for sardine and anchovy in ICES areas VIII and IX, 24–28 October 2005, Vigo, Spain. ICES CM 2006/LRC:01, p. 126.
- IUCN 2006 IUCN red list of threatened species See www.iucnredlist.org
- Josey S.A, Kent E.C, Taylor P.K. New insights into the ocean heat budget closure problem from analysis of the SOC air–sea flux climatology. J. Clim. 1999;12:2856–2880. doi:10.1175/1520-0442(1999)012<2856:NIITOH>2.0.CO;2 [Google Scholar]
- McMahon C.R, Hays G.C. Thermal niche, large-scale movements and implications of climate change for a critically endangered marine vertebrate. Global Change Biol. 2006;12:1330–1338. doi:10.1111/j.1365-2486.2006.01174.x [Google Scholar]
- Oro D, Aguilar J.S, Igual J.M, Louazo M. Modelling demography and extinction risk in the endangered Balearic shearwater. Biol. Conserv. 2004;116:93–102. doi:10.1016/S0006-3207(03)00180-0 [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. doi:10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Perry A.L, Low P.J, Ellis J.R, Reynolds J.D. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1915. doi: 10.1126/science.1111322. doi:10.1126/science.1111322 [DOI] [PubMed] [Google Scholar]
- Poulard J.-C, Blanchard F. The impact of climate change on the fish community structure of the eastern continental shelf of the Bay of Biscay. ICES J. Mar. Sci. 2005;62:1436–1443. doi:10.1016/j.icesjms.2005.04.017 [Google Scholar]
- Rayner N.A, Brohan P, Parker D.E, Folland C.K, Kennedy J.J, Vanicek M, Ansell T, Tett S.F.B. Improved analyses of changes and uncertainties in sea surface temperature measured in situ since the mid-nineteenth century: the HadSST2 dataset. J. Clim. 2006;19:446–469. doi:10.1175/JCLI3637.1 [Google Scholar]
- Reid P.C, Edwards M, Hunt H.G, Warner A.J. Phytoplankton change in the North Atlantic. Nature. 1998;391:546. doi:10.1038/35290 [Google Scholar]
- Richardson A.J, Schoeman D.S. Climate impact on plankton ecosystems in the northeast Atlantic. Science. 2004;305:1609–1612. doi: 10.1126/science.1100958. doi:10.1126/science.1100958 [DOI] [PubMed] [Google Scholar]
- Stebbing A.R.D, Turk S.M.T, Wheeler A, Clark K.R. Immigration of southern fish species to southwest England linked to warming of the North Atlantic (1960–2001) J. Mar. Biol. Assoc. UK. 2002;82:177–180. doi:10.1017/S0025315402005325 [Google Scholar]
- Steele J. Do we know what British ‘soft-plumaged petrels’ are? Br. Birds. 2006;99:404–419. [Google Scholar]
- Thomas C.D, Lennon J.J. Birds extend their ranges northwards. Nature. 1999;399:213. doi:10.1038/20335 [Google Scholar]
- Veit R.R, Pyle P, McGowan J.A. Ocean warming and long-term change in pelagic bird abundance within the California Current System. Mar. Ecol. Prog. Ser. 1996;139:11–18. [Google Scholar]
- Yésou P. Recent changes in the summer distribution of the Balearic shearwater Puffinus mauretanicus off western France. Sci. Mar. 2003;67(Suppl. 2):143–148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.