Abstract
Evolutionary conflict in trait performance under different ecological contexts is common, but may also arise from functional coupling between traits operating within the same context. Orb webs first intercept and then retain insects long enough to be attacked by spiders. Improving either function increases prey capture and they are largely determined by different aspects of web architecture. We manipulated the mesh width of orbs to investigate its effect, along with web size, on prey capture by spiders and found that they functioned independently. Probability of prey capture increased with web size but was not affected by mesh width. Conversely, spiders on narrow-meshed webs were almost three times more likely to capture energetically profitable large insects, which demand greater prey retention. Yet, the two functions are still constrained during web spinning because increasing mesh width maximizes web size and hence interception, while retention is improved by decreasing mesh width because more silk adheres to insects. The architectural coupling between prey interception and retention has probably played a key role in both the macroevolution of orb web shape and the expression of plasticity in the spinning behaviours of spiders.
Keywords: Araneidae, behavioural correlations, phenotypic plasticity, silk, tradeoff, web efficiency
1. Introduction
Evolutionary conflict in organismal performance in different ecological contexts is common (Sih et al. 2004), but also results from functionally coupled traits operating in the same context (Podos & Hendry 2006). The evolutionary success of orb weaving spiders depends on efficient interception and retention of insects by webs, but most insects escape webs before being attacked (Nentwig 1982; Eberhard 1990). Interception and retention are largely determined by different features of orb architecture and hence different spinning behaviours, although improving either would increase foraging success.
Web size, location and orientation determine insect interception (Olive 1980; Opell et al. 2006). Adhesive thread density, or mesh width, only secondarily affects whether small insects pass between silk threads (Herberstein & Heiling 1998). However, webs are not passive filters and instead retain subsets of prey that spiders can attack (Eberhard 1990). Prey retention is determined by how silk absorbs kinetic energy and adheres to struggling insects (Opell & Bond 2001; Blackledge & Zevenbergen 2006). Retention therefore depends upon the densities of adhesive capture threads in webs.
Despite influencing prey capture in different ways, there is substantial opportunity for performance conflicts between prey interception and retention. Tightly spaced capture spirals enhance retention but, for any given quantity of silk, also result in smaller orbs potentially decreasing insect interception. Some spiders spin smaller, narrower-meshed orbs only when sated, possibly an adaptive shift towards the capture of larger prey that require higher retention power (Blackledge & Zevenbergen 2006). Spiders may also trade off mesh width versus size of orbs in response to fluctuations of specific prey (Sandoval 1994; Schneider & Vollrath 1998). Here we manipulate the mesh width of orbs to test its effect, along with web size, on prey capture.
2. Material and methods
We collected adult and penultimate female Argiope aurantia from the Dr Paul E. Martin Centre for Field Studies and Environmental Education (Bath, Ohio, USA). Spiders were placed in 40×40×10 cm screen cages. Spiders spun webs within 1–2 days and could forage freely once the Plexiglas sides were removed. We measured prey capture by weighing spiders (±0.1 mg) before and after 6–8 h foraging bouts (between 10.00 and 18.00 h) because ingested biomass reflects prey consumption better than counts of insects in webs (Tso & Severinghaus 1998; Venner & Casas 2005).We also collected and weighed ‘extra’ prey wrapped in webs at the end of each day. We measured spider carapace widths to calculate body condition as the residuals of initial body mass regressed against carapace width. Size might affect ability to subdue insects and condition might affect motivation to attack.
Each morning, we randomly assigned spiders to experimental or control treatments. We doubled the mesh width of experimental webs by burning every other row of sticky silk using a hot wire (Blackledge & Zevenbergen 2006). Control webs retained their original mesh width. All webs were photographed prior to manipulation and later measured using Image J 1.34s (US National Institutes of Health). Capture area was delimited by the inner and outermost rows of capture spiral and influences insect interception rates (Eberhard 1990; Sherman 1994). Mesh width, the distance between rows of capture silk, was averaged along the vertical axis. Web asymmetry measured the degree to which the lower halves of orbs were larger than the upper, and may influence prey capture because spiders could run down webs faster than up (Heiling & Herberstein 1998). Finally, the number of radii in webs provided a measure of the stiffness and energy-absorbing capacity of webs (Craig 1987).
We placed 53 experimental and 53 control webs in the field between 31 July and 23 August.
3. Results
Pre-manipulation web architecture did not differ between treatments (table 1; MANOVA F4,101=0.054, p=0.99) nor did spider morphology (table 2; MANOVA F3,98=0.29, p=0.75). Value of Δ body mass ranged from −4.82 to +13.92 mg h−1, and 58% of spiders lost weight, suggesting no prey capture (figure 1). The proportion of spiders capturing prey (e.g. positive Δ body mass) did not differ between treatments (G test: G=0.21, d.f.=1). An ANCOVA revealed that ln (Δ body mass h−1) did not differ between experimental and control spiders (F1,103=0.01, p=0.92), but increased with web size (F1,103=8.68, p=0.004). We included web size, radii number, web asymmetry and mesh width in a regression model, along with treatment group as a qualitative variable, to test the ability of web architecture to predict ln (Δ body mass h−1). The model was significant (F5,37=4.8, p<0.0025, R2=0.31), however web size was the only significant predictor of mass gain, a measure of prey capture (figure 2; web size F1,37=19.4, p<0.0001; number of radii F1,37=1.8, p=0.18; web asymmetry F1,37=2.6, p=0.12; mesh width F1,37=0.4, p=0.85). Spider condition, an indicator of potential foraging motivation, was also uncorrelated with body mass gain (F1,40=1.3, p=0.34).
Table 1.
Web architecture prior to manipulation (mean±s.d.).
| control webs (n=53) | altered webs (n=53) | |
|---|---|---|
| mesh width (mm) | 4.4±0.8 | 4.4±0.7 |
| web size (cm2) | 637±187 | 622±181 |
| asymmetry | 0.47±0.16 | 0.48±0.16 |
| no. of radii | 30.3±5.2 | 30.0±5.7 |
Table 2.
Spider morphology prior to foraging (mean±s.d.).
| control webs (n=53) | altered webs (n=53) | |
|---|---|---|
| carapace width (mm) | 2.1±0.3 | 2.0±0.4 |
| initial body mass (mg) | 222±120 | 211±107 |
| initial body condition | 0.03±1.05 | −0.09±0.83 |
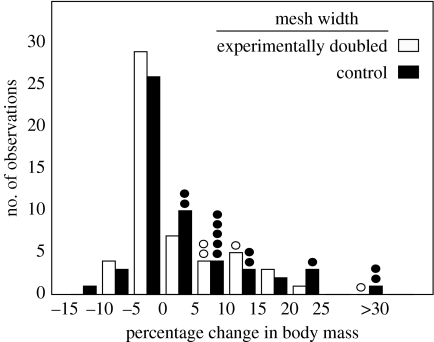
Figure 1.
Mesh width did not affect body mass gain of spiders due to consumption of small prey. Circles indicate percentage ingestible body mass of additional extra prey that were more likely to be wrapped in control webs (open rectangle and circle, experimentally doubled; filled rectangle and circle, control).
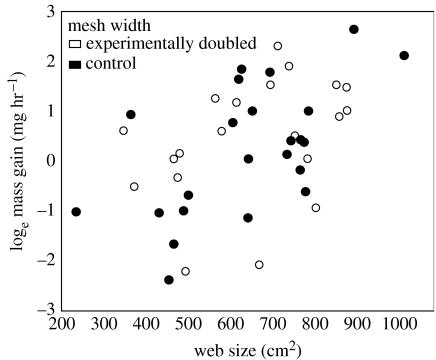
Figure 2.
Spider mass gain increases with web size. Only individuals that captured prey are included (open circle, experimentally doubled; filled circle, control).
Body mass gain measures consumption of common prey, which are often small and contribute little to energetic budgets (Venner & Casas 2005). Large insects, too big to consume during the experiment, remained wrapped in webs at the end of some trials. These extra prey were significantly more common in control versus experimental webs (22.6% versus 7.5%) (G test: G=4.19, d.f.=1, p<0.05) and averaged 10× the mass gained by spiders that completely consumed smaller prey (t-test on natural log, t57=−7.3, p<0.000001). Most extra prey were bees, and all were taxa readily consumed by spiders. They accounted for 57% of all insect biomass, although only 15% of spiders captured them. The related orb spider, Zygiella x-notata, has 78% prey ingestion efficiency (Venner & Casas 2005). After rescaling for ingestion efficiency, extra prey still accounted for more than 50% of all consumable biomass and their capture was solely predicted by treatment group. Spider size, spider condition, web size, number of radii and web asymmetry were all non-significant predictors (F1,94=0.01 to 1.5) when included in a model with mesh width treatment (F1,94=4.9, p<0.029).
4. Discussion
Unexpectedly, mesh width did not affect mass gained by spiders due to the capture of small, common prey (figure 1). However, spiders on narrow-meshed control webs were approximately 3 times more likely to capture rare, large extra prey, and these insects averaged 10 times the mass gained by spiders consuming small prey. Although rare (only 15% of spiders captured them), large insects contributed half of the total consumable biomass of insects captured.
The low rate of prey capture (41% of spiders) probably results in part from spiders not foraging in freely selected sites, but is within the range found in freely foraging Argiope (Horton & Wise 1983; Olive 1980). More than half of all spiders lost 0–5% body mass, probably due to a combination of water loss and basal metabolism and suggesting an upper limit of 0.3±0.02% loss of body mass h−1 (mean±s.e., n=57) for metabolism. This is slightly higher than the approximately 1% loss of body mass day−1 estimated for Z. x-notata ( Venner & Casas 2005) and may result from species variation or shorter acclimation times for our spiders.
Chacón & Eberhard (1980) proposed that wider-meshed webs are energetically more efficient at intercepting prey. Spiders in our experiment were equally likely to capture prey regardless of treatment group. Thus, in theory a spider that spun a web with the wider mesh width of experimental webs could achieve the same probability of capturing prey as control spiders using only half the sticky silk. The positive relationship between web size and mass gain, for both treatment and control spiders (figure 2), supports previous hypotheses that larger orb webs represent greater foraging effort by spiders (Sherman 1994; Venner & Casas 2005). These spiders could also be more motivated to attack. However, the spider condition was not correlated with web size nor did web size correlate with capture of large prey, both of which were expected if attack motivation was more important than web size.
Why do not all spiders spin large, wide-meshed orbs? Venner & Casas (2005) proposed that webs function primarily to capture large, rare insects due to the disproportional energetic gain they represent. More than half of the ingestible insect biomass captured by our spiders consisted of large extra prey that were also rare—captured by only 15% of spiders. The best predictor for their capture was narrow mesh width. This may result from the increased retention power of narrow-meshed orbs because all other measures of web architecture and spider morphology were unrelated to capture of large prey. Mesh width has complex effects on retention of different taxa, but many insects, especially large grasshoppers, escape webs more quickly when it is experimentally increased (Blackledge & Zevenbergen 2006). Thus, selection to improve the capture of energetically profitable large prey could act primarily on the mesh width of webs, independent of other features. Conversely, architectural traits such as web size that influence insect interception may play a greater role in the capture of common, but less profitable, prey. While functionally independent, these two components of prey capture are still constrained behaviourally. For any given amount of silk, spiders may spin either larger orbs that maximize interception of smaller prey or compact webs that maximize retention of larger prey. Thus, spiders face substantial functional tradeoffs when spinning webs, and architectural coupling has probably played a key role in both the macroevolution of orb web shape and the expression of plasticity in spinning behaviours.
Acknowledgments
We thank Peter Niewiarowski and two anonymous reviewers for their insightful comments. This is publication no.14 of The University of Akron Field Station and was conducted under research permit no.2005-003. This research was funded by The National Science Foundation (DEB-0516038).
References
- Blackledge T.A, Zevenbergen J.M. Mesh width influences prey retention in spider orb webs. Ethology. 2006;112:1194–1201. doi:10.1111/j.1439-0310.2006.01277.x [Google Scholar]
- Chacón P, Eberhard W.G. Factors affecting numbers and kinds of prey caught in artificial spider webs, with considerations of how orb webs trap prey. Bull. Br. Arachnol. Soc. 1980;5:29–38. [Google Scholar]
- Craig C.L. The ecological and evolutionary interdependence between web architecture and web silk spun by orb web weaving spiders. Biol. J. Linn. Soc. 1987;30:135–162. [Google Scholar]
- Eberhard W.G. Function and phylogeny of spider webs. Annu. Rev. Ecol. Syst. 1990;21:341–372. doi:10.1146/annurev.es.21.110190.002013 [Google Scholar]
- Heiling A.M, Herberstein M.E. The web of Nuctenea sclopetaria (Araneae, Araneidae): relationship between body size and web design. J. Arachnol. 1998;26:91–96. [Google Scholar]
- Herberstein M.E, Heiling A.M. Does mesh height influence prey length in orb-web spiders (Araneae)? Eur. J. Entomol. 1998;95:367–371. [Google Scholar]
- Horton C.C, Wise D.H. The experimental analysis of competition between two syntopic species of orb web spiders (Araneae, Araneidae) Ecology. 1983;64:929–944. doi:10.2307/1937214 [Google Scholar]
- Nentwig W. Why do certain insects escape from a spider's web? Oecologia (Berlin) 1982;53:412–417. doi: 10.1007/BF00389023. doi:10.1007/BF00389023 [DOI] [PubMed] [Google Scholar]
- Olive C.W. Foraging specializations in orb-weaving spiders. Ecology. 1980;61:1133–1144. doi:10.2307/1936833 [Google Scholar]
- Opell B.D, Bond J.E. Changes in the mechanical properties of capture threads and the evolution of modern orb-weaving spiders. Evol. Ecol. Res. 2001;3:567–581. [Google Scholar]
- Opell B.D, Bond J.E, Warner D.A. The effects of capture spiral composition and orb-web orientation on prey interception. Zoology. 2006;109:339–345. doi: 10.1016/j.zool.2006.04.002. doi:10.1016/j.zool.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Podos J, Hendry A.P. The biomechanics of ecological speciation. In: Herrel A, Speck T, Rowe N.P, editors. Ecology and biomechanics: a mechanical approach to the ecology of animals and plants. CRC Press; Boca Raton, FL: 2006. pp. 301–321. [Google Scholar]
- Sandoval C.P. Plasticity in web design in the spider Parawixia bistriata—a response to variable prey type. Funct. Ecol. 1994;8:701–707. doi:10.2307/2390229 [Google Scholar]
- Schneider J.M, Vollrath F. The effect of prey type on the geometry of the capture web of Araneus diadematus. Naturwissenschaften. 1998;85:391–394. doi:10.1007/s001140050521 [Google Scholar]
- Sherman P.M. The orb web—an energetic and behavioral estimator of a spiders dynamic foraging and reproductive strategies. Anim. Behav. 1994;48:19–34. doi:10.1006/anbe.1994.1208 [Google Scholar]
- Sih A, Bell A.M, Johnson J.C, Ziemba R.E. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 2004;79:241–277. doi: 10.1086/422893. doi:10.1086/422893 [DOI] [PubMed] [Google Scholar]
- Tso I.M, Severinghaus L.L. Ingested biomass of prey as a more accurate estimator of foraging intake by spider predators. J. Arachnol. 1998;26:405–407. [Google Scholar]
- Venner S, Casas J. Spider webs designed for rare but life-saving catches. Proc. R. Soc. B. 2005;272:1587–1592. doi: 10.1098/rspb.2005.3114. doi:10.1098/rspb.2005.3114 [DOI] [PMC free article] [PubMed] [Google Scholar]