Abstract
Sperm have traditionally been regarded as energetically cheap and effectively limitless in supply, although evidence conflicting with this view has become increasingly abundant. For instance, males from a variety of taxa have been shown to strategically partition sperm across ejaculates in response to perceived sperm competition risk. It follows that males might also be predicted to adaptively modulate the rate at which sperm are produced. Here we show that, in the giant sperm producing fruitfly Drosophila bifurca, solitary males with infrequent access to females produce sperm at a much lower rate than males raised in association with females and other males. Our results support the prediction that males with little risk of sperm competition risk or few mating opportunities should divert resources away from gamete production.
Keywords: sperm production rate, sociosexual situation, sperm competition, spermatogenesis, Drosophila bifurca
1. Introduction
Sperm outnumber eggs in every known species, usually by orders of magnitude. Recognition of this pattern inspired the formulation of modern sexual selection theory (Andersson 1994; Shuster & Wade 2003). Bateman (1948) argued that sex differences result from selection on females choosing among competing males and that male reproductive success is limited not by the number of gametes they produce themselves but by their ability to fertilize female gametes, which are relatively rare. Traditionally, sperm have been considered energetically inexpensive and effectively limitless in supply (Bateman 1948; Parker 1982).
Dewsbury (1982) reviewed evidence contradictory to this notion, and additional studies conflicting with the traditional view have become increasingly abundant. First, experiments suggest that sperm production is energetically costly in a variety of taxa (e.g. Van Voorhies 1992; Gage & Cook 1994; Pitnick et al. 1995, 2006; Olsson et al. 1997). Second, studies have demonstrated that male sperm supplies can be limited, with the number of sperm per ejaculate declining with successive copulations (e.g. Nakatsuru & Kramer 1982; Birkhead 1991; Preston et al. 2001; Pattarini et al. 2006). When sperm are costly and limited in availability, selection is predicted to favour males that strategically partition their sperm across ejaculates (Dewsbury 1982). Indeed, in insects, crustaceans, fishes, birds and mammals, males have been observed to tailor the number of sperm per ejaculate in response to perceived risk of sperm competition or perceived female quality (reviewed by Wedell et al. (2002)).
Selection might similarly be expected to favour modulation of the rate at which sperm are produced. When mating opportunities are infrequent or the risk of sperm competition is low, males should decrease investment in sperm production and increase investment in the growth and maintenance of somatic tissue (Parker 1998). Schärer et al. (2004) report a positive relationship between group size and testis size, and between testis size and testicular activity in a hermaphroditic marine flatworm (Macrostomum sp.), suggesting that individuals in this system allocate more resources to sperm production when the risk of sperm competition is high (see also Gage 1995).
We experimentally investigated the extent to which mating frequency and male–male competition influence the rate of sperm production in the fruitfly Drosophila bifurca. Males of this species manufacture the largest sperm on record (nearly 6 cm in length) with testes that comprise approximately 11% of their dry body mass (Pitnick 1996). As a consequence of the energetic demands of producing giant sperm, male D. bifurca produce very few sperm—just 5.8 sperm for every egg produced by females of this species (Bjork & Pitnick 2006). If the adaptive modulation of sperm production rate is to be discovered in additional species, D. bifurca is a probable candidate.
2. Material and methods
Details of the D. bifurca population and scanning electron microscopy techniques are described elsewhere (Bjork & Pitnick 2006). Experimental flies were reared under standardized conditions on cornmeal/agar/molasses medium and live yeast, collected on the day of eclosion, separated by sex following light anesthetization and housed in eight-dram shell vials containing medium and live yeast. On the first day, 120 virgin males were randomly assigned to one of the two treatments—‘infrequent mating’ and ‘frequent mating’ (60 males per treatment)—for seven weeks, at which point sperm production rates were quantified. Males in the infrequent mating treatment were housed individually in vials for the duration of the experiment. These males were provided with one mature, virgin female for 1 h at the end of each week, from week 1 to week 6. The frequent mating vials (20 in total) each contained three males and nine reproductively mature females, thus ensuring that copulation was common for the males in this treatment. All flies were transferred twice weekly to fresh vials. To ensure that males in the frequent mating treatment were given access to a large number of sexual partners, females were rotated among vials at each transfer, and they were replaced with fresh mature, virgin females every third transfer. D. bifurca males attain reproductive maturity at 17-day post-eclosion (Pitnick et al. 1995). Infrequent mating males thus copulated not more than four times (weeks 3–6) before their sperm production rates were quantified. Although mating frequency was not quantified, copulations in the frequent mating treatment vials were regularly observed. Male and female remating in D. bifurca is high, both within and across successive days (Bjork & Pitnick 2006), and all males in the frequent mating treatment probably mated seven or more times per week from maturity to the end of week 7.
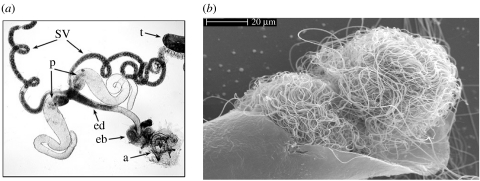
Sperm production rates were determined by depletion of male sperm supply and measuring the replenishment rate. Specifically, males from each treatment were individually placed into vials (each containing five mature, virgin females) to mate. After 2.5 h, all females were removed and one half of the males were immediately frozen (t=0 h). The remaining males were transferred to fresh vials without females and provided an additional 6 h for spermatogenesis before they too were frozen (t=6 h). Reproductive tracts (figure 1a) were dissected from each male and mature sperm (which are individually rolled into compact balls in the paired seminal vesicles; figure 1b) were directly counted using differential interference contrast microscopy. Differences in mean sperm number between t=6 and 0 h were used to estimate sperm production rate for each treatment.
Figure 1.
Drosophila bifurca male reproductive tract. (a) The seminal vesicles (sv) pictured here appear filled to capacity and were dissected from a mature, virgin male whose sperm supply was not diminished (t, testes; p, paragonia; ed, ejaculatory duct; eb, ejaculatory bulb; a, aedeagus). (b) Scanning electron micrograph showing two sperm balls within a dissected seminal vesicle.
3. Results
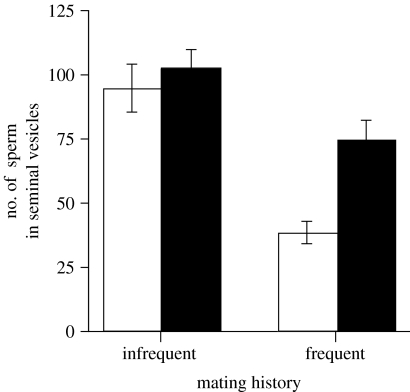
Sperm counts were obtained from 51 infrequent mating and 41 frequent mating males (four males had aberrant testes or were poorly dissected, and 24 died or were lost over the course of the 7-week experiment). Males in the frequent mating treatment were estimated to produce 36.37 sperm over 6 h, versus 8.27 sperm in the infrequent mating treatment (figure 2). In the infrequent mating treatment, the mean number of sperm in the seminal vesicles at t=6 h was not significantly greater than at t=0 h (analysis of variance, ANOVA, F1,49=0.514, p=0.477). However, in the frequent mating treatment, the difference between mean number of sperm at t=6 and 0 h was highly significant (ANOVA, F1,39=17.841, p=0.0001). An additional ANOVA on sperm numbers was performed to test for an interaction between treatment (‘frequent’ and ‘infrequent’) and time (t=0 and 6 h). This interaction was found to be marginally significant (ANOVA, F1,88=3.51, p=0.0645), though when sperm counts were square-root transformed, as recommended by Sokal & Rohlf for ‘count’ data (1981), the interaction was found to be significant (ANOVA, F1,88=4.97, p=0.0284). Interestingly, infrequent mating males were left with more sperm in their seminal vesicles after the ‘sperm diminishment’ stage (at t=0 h) than were frequent mating males (ANOVA, F1,45=25.124, p<0.0001). This does not mean that the sperm depletion technique failed. Infrequent mating males were observed to copulate during the diminishment stage. In addition, their seminal vesicles were not filled to capacity when sperm was counted (i.e. they were not as full as the seminal vesicles in figure 1, which belong to a mature, virgin male and are filled to capacity).
Figure 2.
Effect of mating history on sperm production rate in Drosophila bifurca. The number of sperm in the seminal vesicles of males at t=0 h (white bars) and t=6 h (black bars). Error bars represent 1 s.e.
4. Discussion
We present evidence for the adaptive modulation of sperm production rate in response to sociosexual situation in the fruitfly D. bifurca. This phenomenon has been documented once before, in hermaphroditic flatworms raised in varying group sizes (Schärer et al. 2004). As was the case for the flatworm study, our experimental design prevents us from identifying the specific stimulus (or stimuli) that triggers modulation of sperm production. Our frequent mating and infrequent mating treatments differed according to group size (9 versus 1, respectively), sex ratio (9 : 3 versus 0 : 1), availability of potential mates (continuous versus weekly), exposure to rival males (continuous versus never) and risk of sperm competition (continuous versus never).
Selection may also favour the modulation of sperm production to the extent that it circumvents any negative effects that sperm ageing has on male fitness. Sperm performance and offspring viability have been shown to diminish with increasing sperm age and the number of divisions within the male germ line (reviewed by Siva-Jothy 2000; Radwan 2003). This ‘young sperm advantage’ has been used to provide an adaptive explanation for why male sperm dumping (e.g. masturbation) has evolved (Baker & Bellis 1993). If sperm dumping occurs in D. bifurca, however, it appears to play at most a minor role in its gametic strategy. Infrequent mating males had not mated for 7 days when they began the sperm depletion phase. These males were observed to copulate; old sperm had accumulated and were not dumped (at least not in high numbers), as evidenced by their relatively high sperm stores at the end of diminishment (t=0 h; figure 2).
The higher sperm stores following depletion in the infrequent mating males, relative to the frequent mating males, are not surprising. Given the high risk of sperm competition in the frequent mating treatment, these males would be expected to transfer larger ejaculates (reviewed by Wedell et al. 2002). They would also be expected to replenish their sperm supply at a faster rate, as our data suggest. Additional experiments are required, however, to determine definitively whether the plasticity of sperm production rate is regulated by positive feedback from the males' sociosexual environment (i.e. increased mating frequency or sperm competition risk) or negative feedback from within the individual (i.e. sensory stimuli within the reproductive tract that slow down spermatogenesis when sperm stores are approaching full capacity, as hypothesized by Schärer et al. (2004)).
Acknowledgments
We thank B. A. Byrnes for superb technical assistance, W. J. Etges for directions to the D. bifurca collection site and D. M. Higginson, C. J. Rhodes, R. A. Schmedicke, W. T. Starmer, J. A. C. Uy, L. L. Wolf and two anonymous referees for useful comments. This work was supported by a National Science Foundation grant (DEB-0315008) to S.P. and A.B.
References
- Andersson M.B. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Baker R.R, Bellis M.A. Human sperm competition: ejaculate adjustment by males and the function of masturbation. Anim. Behav. 1993;46:861–885. doi:10.1006/anbe.1993.1271 [Google Scholar]
- Bateman A.J. Intrasexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R. Sperm depletion in the Bengalese finch, Lonchura striata. Behav. Ecol. 1991;2:267–275. doi:10.1093/beheco/2.4.267 [Google Scholar]
- Bjork A, Pitnick S. Intensity of sexual selection along the anisogamy–isogamy continuum. Nature. 2006;441:742–745. doi: 10.1038/nature04683. doi:10.1038/nature04683 [DOI] [PubMed] [Google Scholar]
- Dewsbury D.A. Ejaculate cost and male choice. Am. Nat. 1982;119:601–610. doi:10.1086/283938 [Google Scholar]
- Gage M.J.G. Continuous variation in reproductive strategy as an adaptive response to population density in the moth Plodia interpunctella. Proc. R. Soc. B. 1995;261:25–30. doi:10.1098/rspb.1995.0112 [Google Scholar]
- Gage M.J.G, Cook P.A. Sperm size or numbers? Effects of nutritional stress upon eupyrene and apyrene sperm production strategies in the moth Plodia interpunctella (Lepidoptera: Pyralidae) Funct. Ecol. 1994;8:594–599. doi:10.2307/2389920 [Google Scholar]
- Nakatsuru K, Kramer D.L. Is sperm cheap? Limited male fertility and female choice in the lemon terta (Pisces, Characidae) Science. 1982;216:753–754. doi: 10.1126/science.216.4547.753. doi:10.1126/science.216.4547.753 [DOI] [PubMed] [Google Scholar]
- Olsson M, Madsen T, Shine R. Is sperm really so cheap? Costs of reproduction in male adders, Vipera berus. Proc. R. Soc. B. 1997;264:455–459. doi:10.1098/rspb.1997.0065 [Google Scholar]
- Parker G.A. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 1982;96:281–294. doi: 10.1016/0022-5193(82)90225-9. doi:10.1016/0022-5193(82)90225-9 [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 3–54. [Google Scholar]
- Pattarini J.M, Starmer W.T, Bjork A, Pitnick S. Mechanisms underlying the sperm quality advantage in Drosophila melanogaster. Evolution. 2006;60:2064–2080. doi:10.1111/j.0014-3820.2006.tb01844.x [PubMed] [Google Scholar]
- Pitnick S. Investment in testes and the cost of making long sperm in Drosophila. Am. Nat. 1996;148:57–80. doi:10.1086/285911 [Google Scholar]
- Pitnick S, Markow T.A, Spicer G.S. Delayed male maturity is a cost of producting large sperm in Drosophila. Proc. Natl Acad. Sci. USA. 1995;92:10 614–10 618. doi: 10.1073/pnas.92.23.10614. doi:10.1073/pnas.92.23.10614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, Jones K.E, Wilkinson G.S. Mating system and brain size in bats. Proc. R. Soc. B. 2006;273:719–724. doi: 10.1098/rspb.2005.3367. doi:10.1098/rspb.2005.3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B.T, Stevenson I.R, Pemberton J.M, Wilson K. Dominant rams lose out by sperm depletion. Nature. 2001;409:681–682. doi: 10.1038/35055617. doi:10.1038/35055617 [DOI] [PubMed] [Google Scholar]
- Radwan J. Male age, germline mutations and the benefits of polyandry. Ecol. Lett. 2003;6:581–586. doi:10.1046/j.1461-0248.2003.00484.x [Google Scholar]
- Schärer L, Ladurner P, Rieger R.M. Bigger testes do work more: experimental evidence that testis size reflects testicular cell proliferation activity in the marine invertebrate, the free-living flatworm Macrostomum sp. Behav. Ecol. Sociobiol. 2004;56:420–425. doi:10.1007/s00265-004-0802-9 [Google Scholar]
- Shuster S.M, Wade M.J. Princeton University Press; Princeton, NJ: 2003. Mating systems and strategies. [Google Scholar]
- Siva-Jothy M.T. The young sperm gambit. Ecol. Lett. 2000;3:172–174. doi:10.1046/j.1461-0248.2000.00146.x [Google Scholar]
- Sokal R.R, Rohlf F.J. W. H. Freeman and Company; San Francisco, CA: 1981. Biometry. [Google Scholar]
- Van Voorhies W.A. Production of sperm reduces nematode lifespan. Nature. 1992;360:456–458. doi: 10.1038/360456a0. doi:10.1038/360456a0 [DOI] [PubMed] [Google Scholar]
- Wedell N, Gage M.J.G, Parker G.A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–314. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]