Abstract
Remembering individual identities is part of our own everyday social life. Surprisingly, this ability has recently been shown in two social insects. While paper wasps recognize each other individually through their facial markings, the ant, Pachycondyla villosa, uses chemical cues. In both species, individual recognition is adaptive since it facilitates the maintenance of stable dominance hierarchies among individuals, and thus reduces the cost of conflict within these small societies. Here, we investigated individual recognition in Pachycondyla ants by quantifying the level of aggression between pairs of familiar or unfamiliar queens over time. We show that unrelated founding queens of P. villosa and Pachycondyla inversa store information on the individual identity of other queens and can retrieve it from memory after 24 h of separation. Thus, we have documented for the first time that long-term memory of individual identity is present and functional in ants. This novel finding represents an advance in our understanding of the mechanism determining the evolution of cooperation among unrelated individuals.
Keywords: ants, individual recognition, long-term memory
1. Introduction
Individual recognition is a particular case of discrimination requiring that each individual carries a unique set of recognizable labels. Human social behaviour largely relies upon individual relationships, which can be maintained because we have mental representations of other individuals' faces that are stable over time (Ellis 1992). Other primates that interact repeatedly with one another may also use long-term representations of individual identities (Cheney & Seyfarth 1982). Individual recognition has further been reported in other vertebrates (Thom & Hurst 2004) and in some invertebrates such as crabs (Gherardi & Atema 2005).
In social insects, communication is based on chemicals and there is evidence that the cues mediating social recognition are primarily encoded in the mixture of cuticular hydrocarbons (Lenoir et al. 2001). However, while social insect recognition systems are very sophisticated, they were traditionally assumed to be unsuitable for individual recognition. Ants, for example, live in large cooperative groups, so that individual recognition seems improbable in colonies of hundreds of individuals. Individual identity labels may also be selected against because they would allow the expression of nepotistic traits by full-sib fractions of a large colony (Keller 1997; Boomsma et al. 2003). Common wisdom was therefore that social insects classify conspecifics into simple collective categories such as nestmates, castes or age classes (Hölldobler & Wilson 1990). This is true in most cases. Indeed, individual recognition may occur in social insects only in particular circumstances. It has been shown in small societies of paper wasps (Tibbetts 2002), based on visual colour patterns, and among co-founding queens of the ant, Pachycondyla villosa (D'Ettorre & Heinze 2005), based on chemical cues. Both systems are characterized by long-term stable dominance hierarchies enforced by individual aggression. In Pachycondyla, associations of genetically unrelated founding queens may give rise to multiple queen mature societies (Heinze et al. 2001), contrary to other ant species where all but one queen is eliminated after the emergence of workers (Bernasconi & Strassmann 1999). We predicted that individual recognition should require long-term memory (24 h memory; cf. Menzel 1999) of individual identity, and here, we tested whether such memory occurred and whether it is robust enough not to be erased by interactions with different individuals.
2. Material and methods
Natural founding associations of P. villosa and P. inversa are typically formed by two or more queens who establish a dominance hierarchy with division of labour (Heinze et al. 2001; D'Ettorre et al. 2005). Queen associations were collected in a cocoa plantation (Brazil): a total of 44 P. villosa and 24 P. inversa founding queens (see electronic supplementary material).
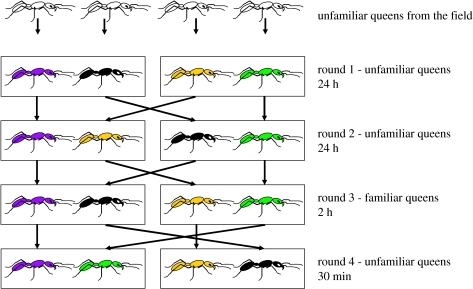
Each experimental session consisted of four queens encountering each other one-to-one in four consecutive rounds (figure 1), in each of which the aggression level was quantified. Queens in a given session came from four different natural associations and participated only in that particular session. In the first round (24 h), paired queens were unfamiliar with each other, as they were in the second round (24 h). But in the third round (2 h), each queen met the same individual as in the first round, so they were familiar (here ‘familiar’ means queens shared a nest box for 24 h during the first round of the experiment). The fourth round (30 min) of interactions was again between different unfamiliar queens, enabling us to show that queens were still motivated to be aggressive. Aggression was observed during the first 10 min of each round. Behaviours used to quantify aggression were antennal contact, mandible opening, biting and stinging, which were combined into a single aggression index (see electronic supplementary material). Each encounter was in a new clean plastic box. This design allowed us to: (i) test whether queens expressed long-term (24 h) memory of previous opponents and its strength, and (ii) eliminate any effect of possible exchange of recognition cues between queens, because if such exchanges were happening they would similarly affect the results in each round. We carried out 11 experimental sessions for P. villosa and 6 for P. inversa, involving 44 and 24 queens, respectively. Friedman analysis of variance was used to compare the aggression index among rounds (Statistica v. 6, StatSoft), followed by multiple comparisons (Siegel & Castellan Jr 1988).
Figure 1.
Schematic set-up showing the design of one experimental session to test individual recognition and long-term memory in Pachycondyla ants. The session involved four different queens encountering each other one-to-one in four consecutive rounds. Arrows indicate how queens were moved between rounds: each queen met an unfamiliar individual in rounds 1, 2 and 4, and a familiar individual in round 3. The pairs of queens were housed in clean plastic boxes in each round to eliminate the possibility of residual cues influencing their behaviour.
Pachycondyla villosa and P. inversa queens show similar colony founding strategies but different behaviours during hierarchy establishment: overt aggression in P. villosa (biting and stinging attempts) and ritualized aggression in P. inversa. Here queens engage in a ‘dance’, with mandible opening and short bites in each other's mandibles, until one of them assumes a submissive posture (Kolmer & Heinze 2000). We thus obtained unambiguous information on social status of P. inversa queens by additional observations during the different rounds. In round 2, queens that were dominant in the first round were always paired with queens that were subordinate.
Pachycondyla villosa queens use chemical cues for individual recognition, and their cuticular chemical profile is not associated with dominance (D'Ettorre & Heinze 2005). To investigate whether the same applies to P. inversa, we studied the chemical profile of the 24 queens after the behavioural experiment (see electronic supplementary material).
3. Results and discussion
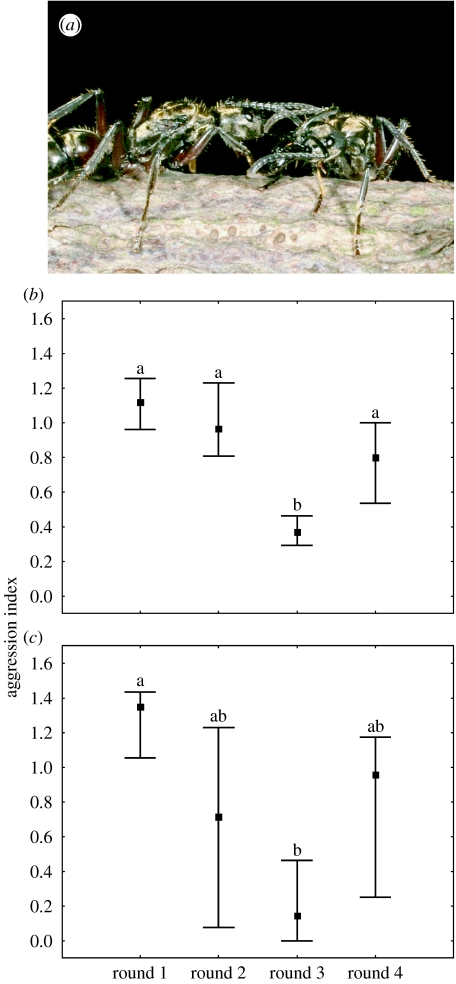
We found a highly significant difference in the aggression index among rounds for both species (figure 2; Friedman ANOVA, F3,22=40.42, p<0.01; F3,12=16.04, p<0.01, for P. villosa and P. inversa, respectively). For P. villosa, aggression was lower when queens were returned to a familiar individual: the third round differed significantly from all other rounds (figure 2b, p<0.05 in all cases). The same trend was observed in P. inversa, but here only rounds 1 and 3 differed significantly (figure 2c). This is indeed the crucial difference, namely that queens were less aggressive when familiar. In both species, we observed a decrease in aggression after round 1, which might be due to experimental manipulation. Nonetheless, aggression was higher in round 4 than in round 3, showing that queens were still motivated to be aggressive when meeting an unfamiliar opponent.
Figure 2.
(a) Two familiar queens of Pachycondyla villosa interacting peacefully. Aggression index in (b) P. villosa and (c) P. inversa in the four rounds of dyadic encounters between queens (Friedman ANOVA, F3,22=40.42, p<0.01 and F3,12=16.04, p<0.01, for P. villosa and P. inversa, respectively). Median, 25 and 75% percentiles are shown. Different letters indicate significant differences between rounds (multiple comparisons; Siegel & Castellan Jr 1988).
We expected familiar individuals not having to re-establish dominance hierarchies when meeting again. In round 3, indeed, 22 out of 24 (92%) P. inversa queens maintained their initial status established in round 1 (expected 100%). But queens should establish a new rank order when meeting an unfamiliar individual in round 2. Out of 24, 10 (42%) queens changed their status between rounds 1 and 2 (expected 50%, when status is changed by chance). Thus, a significantly larger proportion of queens changed their status in round 2 than in round 3 (McNemar test, Χ2=4.9, p=0.026), indicating that queens do not merely recognize the social status of their opponent (status badges, Tibbetts & Dale 2004). To substantiate this finding, we investigated whether the possible variation in the chemical profile of P. inversa queens would allow differentiation among individuals according to their social status. The chemical profiles of P. inversa queens are characterized by a mixture of hydrocarbons in variable proportions (figure 1 in the electronic supplementary material). A principal component analysis of the queens' profiles produced six principal components, which explained 89.06% of the total variance. We did not observe any enhanced chemical similarity between queens with identical social status: the groups of dominants and subordinates were not differentiated by a discriminant analysis based on the PCA factor scores (Wilks' Lambda =0.836, F6,17=0.553, p=0.760).
Taken together, our results show that queen–queen recognition in these two ant species is based on long-term memory of individual identity. In particular, 24 h of separation does not affect the memory of a previously encountered individual. Furthermore, this long-term memory is not affected by an in-between interaction with a different individual, which suggests that retroactive interference (cf. Anderson 1995) does not seem to occur in this particular social context. Memories evolve in time and are based on dynamic processes that start at the very moment in which a given experience is collected. We observed that the dominance hierarchy between queens in the first round of interactions was already established within the first 6 h of cohabitation, when aggression disappeared. This indicates that the memory of individual identity should last at least 42 h in our experiments.
Recent studies using operant conditioning paradigm showed that volatile olfactory cues are individually learned and remembered by ant workers for at least 5 min (Dupuy et al. 2006). Memory used in navigation has also been documented in ants (Collett & Collett 2002). In both cases, the behavioural context is very different from recognition of individual identity in queens. Memory dynamics and learning abilities are well studied in honeybees at the behavioural, cellular and molecular level. Despite their developed cognitive abilities (Menzel 1999), honeybees may also show memory constraints (Benard & Giurfa 2004), suggesting that the maintenance of accurate memories is possibly an active and costly process (Dukas 1999). The dynamics and optimal level of memory for a particular social or environmental condition may be shaped by its fitness consequences, thus species- and task-specific memory adaptations are expected to be highly variable in their evolutionary constraints. Memory of individual identity is particularly advantageous when repeated contacts among a small number of individuals are likely to occur, a social context which selected for accurate memory in another invertebrate, the hermit crab, where interactions with different individuals do not erase the memory of a former rival (Gherardi & Atema 2005).
For an ant queen, an unsuccessful start at the critical stage of colony founding would have drastic fitness consequences. Multiple queen associations allow faster colony growth because more workers can be produced per unit of time (Hölldobler & Wilson 1990), but they also entail the regulation of costly social conflicts over reproduction. The stable dominance hierarchies with a clear division of labour that Pachycondyla queens establish help to resolve this conflict: dominant queens stay in the nest and subordinates forage. Co-founding queens may thus be separated from each other for relatively long time. Meanwhile, queens in established associations may be confronted with unfamiliar queens trying to join (D'Ettorre et al. 2005), because new Pachycondyla queens are produced year-round. Long-term memory of individual identity appears to be a suitable mechanism to minimize both the cost of repeated assessments during aggressive contest, and the risk of failing to recognize a partner with whom a stable relationship has been established. This novel finding opens up new avenues for focused experimental research on the cognitive abilities of social insects and represents a significant advance in our understanding of mechanisms affecting the evolution of sociality in absence of kin selection. We predict that long-term memory of individual identity is a necessary step for the evolution of cooperation between unrelated individuals regulated by dominance hierarchy.
Acknowledgments
We thank Jacques Delabie, Riviane da Hora and their assistants for their support at the Brazilian Centre for Cocoa Research. Thanks to the Copenhagen Centre for Social Evolution, David Hughes, Fernando Guerrieri, and especially Koos Boomsma and David Nash for their discussions. David Nash provided the photograph in figure 2. Gösta Nachman, Maurice Roux and Thorsten Balsby helped with the statistics. This work was financed by EU-Marie Curie Excellence Grant, EXT-CT-2004-014202 to P.D.E.
Supplementary Material
Electronic supplementary material and methods
Electronic supplementary material figure S1
Pachycondyla queen
Pachycondyla queen with eggs
References
- Anderson J.R. Wiley; New York, NY: 1995. Learning and memory: an integrated approach. [Google Scholar]
- Benard J, Giurfa M. A test of transitive inferences in free-flying honeybees: unsuccessful performance due to memory constraints. Learn. Mem. 2004;11:328–336. doi: 10.1101/lm.72204. doi:10.1101/lm.72204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi B, Strassmann J.E. Cooperation among unrelated individuals: the ant foundress case. Trends Ecol. Evol. 1999;14:477–482. doi: 10.1016/s0169-5347(99)01722-x. doi:10.1016/S0169-5347(99)01722-X [DOI] [PubMed] [Google Scholar]
- Boomsma J.J, Nielsen J, Sundström L, Oldham N.J, Tentschert J, Petersen H.C, Morgan E.D. Informational constraints on optimal sex allocation in ants. Proc. Natl Acad. Sci. USA. 2003;100:8799–8804. doi: 10.1073/pnas.1430283100. doi:10.1073/pnas.1430283100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney D.L, Seyfarth R.M. Recognition of individuals within and between groups of free-ranging vervet monkeys. Am. Zool. 1982;22:519–529. [Google Scholar]
- Collett T, Collett M. Memory use in insect visual navigation. Nat. Rev. Neurosci. 2002;3:542–552. doi: 10.1038/nrn872. doi:10.1038/nrn872 [DOI] [PubMed] [Google Scholar]
- D'Ettorre P, Heinze J. Individual recognition in ant queens. Curr. Biol. 2005;15:2170–2174. doi: 10.1016/j.cub.2005.10.067. doi:10.1016/j.cub.2005.10.067 [DOI] [PubMed] [Google Scholar]
- D'Ettorre P, Kellner K, Delabie J.H.C, Heinze J. Number of queens in founding associations of the ponerine ant Pachycondyla villosa. Insectes Soc. 2005;52:327–332. doi:10.1007/s00040-005-0815-z [Google Scholar]
- Dukas R. Costs of memory: ideas and predictions. J. Theor. Biol. 1999;197:41–50. doi: 10.1006/jtbi.1998.0856. doi:10.1006/jtbi.1998.0856 [DOI] [PubMed] [Google Scholar]
- Dupuy F, Sandoz J.C, Giurfa M, Josens R. Individual olfactory learning in Camponotus ants. Anim. Behav. 2006;72:1081–1091. doi:10.1016/j.anbehav.2006.03.011 [Google Scholar]
- Ellis A.W. Cognitive mechanisms of face processing. Phil. Trans. R. Soc. B. 1992;335:113–119. doi: 10.1098/rstb.1992.0014. doi:10.1098/rstb.1992.0014 [DOI] [PubMed] [Google Scholar]
- Gherardi F, Atema J. Memory of social partners in hermit crab dominance. Ethololgy. 2005;111:271–285. doi:10.1111/j.1439-0310.2004.01060.x [Google Scholar]
- Heinze J, Trunzer B, Hölldobler B, Delabie J.H.C. Reproductive skew and queen relatedness in an ant with primary polygyny. Insectes Soc. 2001;48:149–153. doi:10.1007/PL00001758 [Google Scholar]
- Hölldobler B, Wilson E.O. Harvard University Press; Cambridge, MA: 1990. The ants. [Google Scholar]
- Keller L. Indiscriminate altruism: unduly nice parents and siblings. Trends Ecol. Evol. 1997;12:99–103. doi: 10.1016/s0169-5347(96)10065-3. doi:10.1016/S0169-5347(96)10065-3 [DOI] [PubMed] [Google Scholar]
- Kolmer K, Heinze J. Rank orders and division of labour among unrelated cofounding ant queens. Proc. R. Soc. B. 2000;267:1729–1734. doi: 10.1098/rspb.2000.1202. doi:10.1098/rspb.2000.1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir A, D'Ettorre P, Errard C, Hefetz A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001;46:573–599. doi: 10.1146/annurev.ento.46.1.573. doi:10.1146/annurev.ento.46.1.573 [DOI] [PubMed] [Google Scholar]
- Menzel R. Memory dynamics in the honeybee. J. Comp. Physiol. A. 1999;185:323–340. doi:10.1007/s003590050392 [Google Scholar]
- Siegel S, Castellan N.J., Jr . 2nd edn. McGraw-Hill College; Columbus, OH: 1988. Nonparametric statistics for the behavioral sciences. [Google Scholar]
- Thom M.D, Hurst J.L. Individual recognition by scent. Ann. Zool. Fenn. 2004;41:765–788. [Google Scholar]
- Tibbetts E.A. Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. B. 2002;269:1423–1428. doi: 10.1098/rspb.2002.2031. doi:10.1098/rspb.2002.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts E.A, Dale J. A socially enforced signal of quality in a paper wasp. Nature. 2004;432:218–222. doi: 10.1038/nature02949. doi:10.1038/nature02949 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material and methods
Electronic supplementary material figure S1
Pachycondyla queen
Pachycondyla queen with eggs