Abstract
When glaciers retreat they expose barren substrates that become colonized by organisms, beginning the process of primary succession. Recent studies reveal that heterotrophic microbial communities occur in newly exposed glacial substrates before autotrophic succession begins. This raises questions about how heterotrophic microbial communities function in the absence of carbon inputs from autotrophs. We measured patterns of soil organic matter development and changes in microbial community composition and carbon use along a 150-year chronosequence of a retreating glacier in the Austrian Alps. We found that soil microbial communities of recently deglaciated terrain differed markedly from those of later successional stages, being of lower biomass and higher abundance of bacteria relative to fungi. Moreover, we found that these initial microbial communities used ancient and recalcitrant carbon as an energy source, along with modern carbon. Only after more than 50 years of organic matter accumulation did the soil microbial community change to one supported primarily by modern carbon, most likely from recent plant production. Our findings suggest the existence of an initial stage of heterotrophic microbial community development that precedes autotrophic community assembly and is sustained, in part, by ancient carbon.
Keywords: microbial communities, organic matter, carbon, chronosequence
1. Introduction
The global retreat of glaciers is receiving much attention, especially as a signal of climate change (Oerlemans 2005). When glaciers retreat they expose barren substrates that become colonized by organisms, beginning the process of primary succession. The common view is that primary succession is started by autotrophs, including algae, mosses, lichens and higher plants, owing to a lack of carbon for heterotrophic organisms (Walker & del Moral 2003). Fixation of carbon by autotrophs then leads to the build-up of organic matter providing resources for the development of heterotrophic communities, which drive ecosystem processes of decomposition and nutrient cycling (Bardgett & Walker 2004; Bardgett et al. 2005). Recent studies, however, show that diverse heterotrophic microbial communities occur in newly exposed glacial substrates before autotrophic communities have established (Tscherko et al. 2003). This study aimed to examine how heterotrophic microbial communities of recently deglaciated terrain function in the absence of carbon inputs from autotrophs, and how microbial carbon use changes as succession proceeds and autotroph-derived organic matter accumulates in soil. This was tested by measuring patterns of soil organic matter development and changes in microbial community composition and carbon use along a 150-year chronosequence of a retreating glacier in the Austrian Alps.
2. Material and methods
We sampled soils from the foreland of the Ödenwinkelkees glacier (47°06′ N, 12°39′ E), above the treeline (2050–2200 m) in the Austrian Alps. The glacier has been retreating since 1850 at an average rate of 10 m yr−1, creating a chronosequence of soil development over 1.5 km. The glacial moraine is composed of feldspathic rocks, mica schist and gneiss; soils are leptic regosols. Soil was sampled from five sites along the chronosequence, which had been ice free for 3, 18, 49, 101 and 145 years. These sites varied in size according to the terrain and were between 25×20 m (101 years ice free) and 10×10 m (18 years ice free). Within each site, six discrete sampling patches (1 m2) were randomly selected and a composite soil sample was collected (0–10 cm) from each; these samples were considered statistically independent. Organic matter was collected from sediments below the glacier, from its surface (cryoconite) and slopes of the glacial valley. All analyses were done on replicate samples (n=6), except for 14C and thermal analysis, which were done on a bulked sample from each site.
Total soil C was measured by elemental analysis and soil microbial community biomass and composition were assessed using phospholipid fatty acid analysis (PLFA; Bardgett et al. 1996). PLFAs used to represent bacteria were i15:0, a15:0, 15:0, i16:0, 16:1ω9, i17:0, cy17:0, 17:0, 18:1ω9c, 18:1ω9t and cy19:0, and 18:2ω6 was used to represent fungi (Bardgett et al. 1996). For isotope analysis, samples were first washed with acid (1 M HCl) to remove potential traces of carbonate before converting the organic carbon to CO2 in a high-pressure combustion vessel (Harkness & Wilson 1972). Soil was also quantitatively separated into three types of recalcitrant organic matter, namely humic acid (alkali soluble and acid insoluble), fulvic acid (acid soluble) and humin fractions (alkali and acid insoluble), using procedures based on Shore et al. (1995); these were analysed for their 13C and 14C content. Respiration measurements were done on fresh, root-free soil and respired CO2 was collected on cartridges containing molecular sieves and recovered by heating (500°C; Hardie et al. 2005). All recovered CO2 samples were cryogenically purified and subsamples were taken for 13C measurement by isotope ratio mass spectrometry (IRMS), with results expressed relative to the international Vienna Pee Dee Belemnite standard. 14C analysis was performed on a CO2 subsample by (depending on sample size) either (i) conversion to graphite and measurement by accelerator mass spectrometry (Xu et al. 2004) or (ii) conversion to benzene and measurement by liquid scintillation counting (Harkness & Wilson 1972). 14C results are expressed as % modern or conventional radiocarbon ages (14C age; Stuiver & Polach 1977). Per cent modern values above 100 show incorporation of radiocarbon produced from the atmospheric testing of nuclear weapons (bomb-14C) and thus unequivocally indicate contributions from carbon fixed post-AD 1955 (Levin & Hesshaimer 2000).
The proportion of recalcitrant organic matter, measured as weight loss due to thermal decomposition of discrete components of organic matter, was determined by thermal analysis (thermogravimetry and differential scanning calorimetry) using a Netzsch thermal analyser STA 449C Jupiter (Lopez Capel et al. 2005).
3. Results
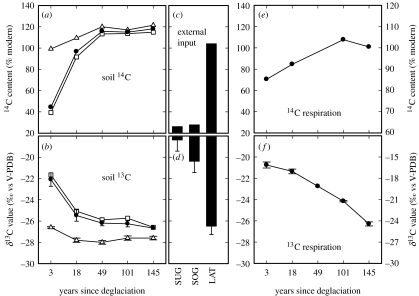
Very little (less than 0.1 mg g−1) organic carbon was detected in substrates recently exposed by glacial retreat (table 1), and the carbon that was present was isotopically distinct from that found in older sites (figure 1b). Radiocarbon dating of soil organic matter of older sites (more than 50 years ice free) revealed that it was relatively young, being derived from recent plant inputs (>100% modern) (figure 1a). In contrast, organic matter of the youngest site was significantly enriched in 13C, relative to that of the older sites, with an average radiocarbon age of more than 7000 years, suggesting that it was derived from ancient carbon (figure 1a,b).
Table 1.
Changes in soil carbon content and organic matter quality with substrate age. (Values in parentheses are standard errors (n=6).)
| years ice free | |||||
|---|---|---|---|---|---|
| 3 | 18 | 49 | 101 | 145 | |
| total C (% DM) | 0.094 (0.03) | 0.22 (0.06) | 0.51 (0.27) | 0.90 (0.25) | 1.12 (0.39) |
| humin (% OM) | 91.0 | 70.0 | 62.7 | 62.2 | 47.9 |
| recalcitrant organic matter by thermal analysis (% OM) | 58.8 | 43.2 | 38.6 | 31.5 | 26.9 |
Figure 1.
Isotopic patterns in organic carbon with terrain age and potential carbon inputs. Data shown are as follows: (a) radiocarbon content (14C in % modern) of soil and (c) potential carbon inputs, including organic material under the glacier (SUG), on the glacier surface (cryoconite; SOG) and organic carbon from soils of lateral slopes (LAT); (b) stable (13C) carbon isotope ratios of soil and (d) potential carbon inputs; (e) radiocarbon and (f) stable isotope composition of soil respired CO2. (a,b) Filled circles, bulk soil; triangles, humic acid fraction; open squares, humin fraction. All 13C data are means±1 s.d. (n=6), expressed relative to the international Vienna Pee Dee Belemnite (V-PDB). For 14C analysis, the six replicates were pooled and analysed as a composite sample.
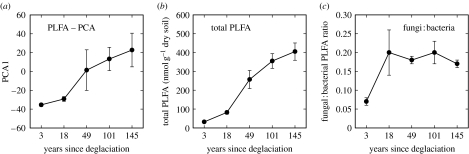
The soil of the recently deglaciated site had a much greater humin concentration than that of older sites, indicating that the ancient carbon was mostly recalcitrant (table 1). Thermal analysis demonstrated that 58.8% of the organic carbon of the youngest site was recalcitrant, declining to 26.9% at the oldest site (table 1). The average 14C age of carbon respired by heterotrophic microbes was 1282 and 644 years for the 3- and 18-year ice-free sites, respectively, whereas at the older sites (i.e. more than 50 years ice free) respired carbon was derived primarily from modern sources, most likely from recent plant production, but potentially also carbon fixed by cyanobacteria (figure 1e,f). Ordination analysis of PLFA data revealed that microbial communities of the two younger sites were significantly (F4,24=24.1, p<0.0001) different from those of older (i.e. more than 50 years ice free) sites (figure 2a). The size of the microbial biomass, measured as total PLFA (F4,24, p<0.0001), and the fungal-to-bacterial PLFA ratio (F4,24, p<0.02), a measure of the abundance or fungi relative to bacteria, were at their lowest at the youngest site (figure 2b,c).
Figure 2.
Patterns of microbial community composition with terrain age. (a) Microbial community composition analysed by principal components analysis (PCA). Data are PCA axis 1 for each site (mean±1 s.d.). PCA1 explains 95.3% of the variation in microbial community composition and differs significantly with terrain age (ANOVA, F5,29=25.2, p<0.0001). PCA1 was positively correlated with an increase in all individual PLFAs (r=0.838–0.996) and hence represents an increase in total PLFA. (b) Total PLFA (nmol g−1 dry soil) and (c) fungal-to-bacterial PLFA ratio.
4. Discussion
Recently exposed glacial substrates are typically skeletal in nature and have negligible organic matter contents (Bardgett & Walker 2004). Consistent with this, we found little carbon (less than 0.1 mg g−1) in recently exposed glacial substrates. The carbon that was present, however, was chemically distinct from that of sites that had been ice free for more than 50 years, being much older, with an average radiocarbon age more than 7000 years, and dominated by recalcitrant organic matter. The organic matter of older sites was more abundant and younger, with a lower proportion of recalcitrant carbon. This build-up of organic matter is typical of primary succession, reflecting the increasing input of litter (root and shoot) from the developing plant community and the fixation of carbon by cyanobacteria (Walker & del Moral 2003). Probable sources of the old carbon in recently exposed sites were organic matter that was previously beneath the glacier (figure 1c,d) and that from the surface of the glacier (cryoconite). While both these sources had approximately the same carbon isotope signature, they are likely to have different origins. Organic matter beneath the glacier most likely contains remnants of preglacial soils and/or geological carbon, whereas that on the glacier surface might include 14C-dead carbon from fossil fuel burning (black carbon) and modern carbon, such as algal-derived carbon (Kaštovská et al. 2006).
While little carbon was present in recently exposed glacial substrates, it supported a functioning microbial community, as evidenced by the detection of fatty acids from living bacteria and fungi. Moreover, the microbial community composition of recently exposed substrates differed from that of older substrates with a significantly greater abundance of bacteria relative to fungi. This was evidenced by the relatively low fungal-to-bacterial PLFA ratio of the youngest site, which subsequently increased along the chronosequence; this pattern is consistent with the idea that the abundance of fungi relative bacteria within the soil microbial community increases as succession proceeds and resources become more available (Bardgett et al. 2005). The average age of carbon respired by microbes of the two youngest sites was relatively old. In contrast, respired carbon of older sites was derived primarily from modern sources, most likely from recent plant production. These data suggest that ancient and recalcitrant carbon, along with modern carbon (e.g. cyanobacteria fixed carbon), sustains the microbial community of recently exposed glacial substrates, whereas microbial communities of older sites are sustained mainly by modern carbon from recent plant production. Ancient carbon, along with modern sources, has also been shown to sustain heterotrophic microbial communities in low carbon ecosystems of Antarctica (Hopkins et al. 2006). Our findings highlight the significance of this carbon source for microbial functioning in low carbon alpine environments.
Collectively, our data provide new insights into the functioning of microbial communities during early stages of succession in glacial forelands, highlighting the role of old and recalcitrant carbon as an energy source in these low carbon environments. Our data are also of relevance for understanding primary community assembly, showing that community assembly by autotrophs is preceded by an initial heterotrophic phase (Hodkinson et al. 2002).
Acknowledgments
We thank David Wardle, David Hopkins and Hana Šantrůčková, and two anonymous referees for their helpful comments on the manuscript. This work was supported by NERC and BBSRC.
References
- Bardgett R.D, Walker L.R. Impact of coloniser plant species on the development of decomposer microbial communities following deglaciation. Soil Biol. Biochem. 2004;36:555–559. doi:10.1016/j.soilbio.2003.11.002 [Google Scholar]
- Bardgett R.D, Hobbs P.J, Frostegård Å. Changes in fungal: bacterial biomass ratios following reductions in the intensity of management on an upland grassland. Biol. Fertil. Soils. 1996;22:261–264. [Google Scholar]
- Bardgett R.D, Bowman W.D, Kaufmann R, Schmidt S.K. A temporal approach to linking aboveground and belowground ecology. Trends Evol. Ecol. 2005;20:634–641. doi: 10.1016/j.tree.2005.08.005. doi:10.1016/j.tree.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Hardie S.M.L, Garnett M.H, Fallick A.E, Rowland A.P, Ostle N.J. Carbon dioxide capture using a zeolite molecular sieve sampling system for isotopic studies (C-13 and C-14) of respiration. Radiocarbon. 2005;47:441–451. [Google Scholar]
- Harkness, D. D. & Wilson, H. W. 1972 Some applications in radiocarbon measurement at the Scottish Research Reactor Centre. In Proc. 8th Int. Radiocarbon Conference, Wellington, New Zealand, October 1972, pp. B101–B115. New Zealand, Wellington: Royal Society.
- Hodkinson I.D, Webb N.R, Coulsen S.J. Primary community assembly on land—the missing stages: why are the heterotrophic organisms always there first? J. Ecol. 2002;90:569–577. doi:10.1046/j.1365-2745.2002.00696.x [Google Scholar]
- Hopkins D.W, Sparrow A.D, Novis P.M, Gregorich E.G, Elberling B, Greenfield L.G. Controls on the distribution of productivity and organic resources in Antarctic Dry Valley soils. Proc. R. Soc. B. 2006;273:2687–2695. doi: 10.1098/rspb.2006.3595. doi:10.1098/rspb.2006.3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaštovská K, Stibal M, Šabacká M, Černá B, Šantrůčková H, Elster J. Microbial community structure and ecology of subglacial sediments in two polythermal Svalbard glaciers characterized by epifluorescence microscopy and PLFA. Polar. Biol. 2006;30:277–287. doi:10.1007/s00300-006-0181-y [Google Scholar]
- Levin I, Hesshaimer V.I. Radiocarbon—a unique tracer of global carbon cycle dynamics. Radiocarbon. 2000;42:69–80. [Google Scholar]
- Lopez Capel E, Sohi S.P, Gaunt J.L, Manning D.A.C. Use of thermogravimetry-differential scanning calorimetry to characterize modelable soil organic matter fractions. Soil Sci. Soc. Am. J. 2005;69:136–140. [Google Scholar]
- Oerlemans J. Extracting a climate signal from 169 glacier records. Science. 2005;308:675–677. doi: 10.1126/science.1107046. doi:10.1126/science.1107046 [DOI] [PubMed] [Google Scholar]
- Shore J.S, Bartley D.D, Harkness D.D. Problems encountered with the 14C dating of peat. Q. Sci. Rev. 1995;14:373–383. doi:10.1016/0277-3791(95)00031-3 [Google Scholar]
- Stuiver M, Polach H.A. Reporting of C-14 data—discussion. Radiocarbon. 1977;19:355–363. [Google Scholar]
- Tscherko D, Rustemeier J, Richter A, Wanek W, Kandeler E. Functional diversity of the soil microflora in primary succession across two glacier forelands. Eur. J. Soil Sci. 2003;54:685–696. doi:10.1046/j.1351-0754.2003.0570.x [Google Scholar]
- Walker L.R, del Moral R. Cambridge University Press; Cambridge, UK: 2003. Primary succession and ecosystem rehabilitation. [Google Scholar]
- Xu S, Anderson R, Bryant C, Cook G.T, Dougans A, Freeman S, Naysmith P, Schnabel C, Scott E.M. Capabilities of the new suerc 5MV AMS facility for C-14 dating. Radiocarbon. 2004;46:59–64. [Google Scholar]