Abstract
Despite the considerable variation in milk composition found among mammals, a constituent common across all groups is lactose, the main sugar and osmole in most eutherians milk. Exceptions to this are the families Otariidae (fur seals and sea lions) and Odobenidae (walruses), where lactose has not been detected. We investigated the molecular basis for this by cloning α-lactalbumin, the modifier protein of the lactose synthase complex. A mutation was observed which, in addition to preventing lactose production, may enable otariids to maintain lactation despite the extremely long inter-suckling intervals during the mother's time at sea foraging (more than 23 days in some species).
Keywords: α-lactalbumin, lactose, lactation, pinnipeds
1. Introduction
Lactose is the major determinant of milk volume as it is unable to diffuse through cell membranes causing influx of water into mammary alveoli to maintain osmotic balance. Consequently, total solids and lactose are generally negatively correlated in mammalian milk (Mepham 1987). For aquatic mammals, milk rich in fat is beneficial to the offspring, providing rapid transfer of energy to the young and, correspondingly, many produce milk low in lactose and rich in fat (Mepham 1987). Within the Pinnipedia (seals, sea lions and walruses), lactose has been detected at trace concentrations in the milk of true seals (Phocidae; Pilson 1965; Urashima et al. 2001), but appears to be absent from the milk of fur seals and sea lions (Otariidae) and walruses (Odobenidae; Pilson 1965; Fay 1982; Urashima et al. 2001). Lactose synthase, the enzyme responsible for producing lactose, consists of two components; the ubiquitously expressed β1,4-galactosyltransferase (β-GT) and the mammary-specific α-lactalbumin (α-LA). It has been suggested that the absence of lactose in the milk of otariids and odobenids is due to the absence of the α-LA gene (Johnson et al. 1972; Urashima et al. 2001), but definitive evidence is lacking. Hence, to determine the molecular basis for the lack of lactose in the milk of otariids and odobenids, we first established the presence of α-LA in these species.
2. Material and methods
Total RNA was extracted from Cape fur seal (Arctocephalus pusillus pusillus) mammary gland tissue and milk collected from subantarctic fur seal (Arctocephalus tropicalis), California sea lion (Zalophus californianus californianus) and Weddell seal (Leptonychotes weddellii) using the Lipid RNeasy Tissue kit (QIAGEN). Oligonucleotide primers (5′-CAGTGGTTATGATACACAA-3′ and 5′-CACAGGAGATGTCACAGA-3′) were designed to conserved internal regions of α-LA cDNA homologues obtained from GenBank (further details described in the electronic supplementary material) and used to amplify Cape fur seal cDNA sequences. Full-length sequences were obtained by screening a lactating Cape fur seal cDNA library with gene-specific and M13 forward or M13 reverse primers. Primers were designed to the 5′ and 3′ ends of the fur seal α-LA cDNA (5′-CAAAATGATGTCCTTTGTC-3′ and 5′-CCTTTATTCAAGACAGAGGT-3′) and used to amplify α-LA from subantarctic fur seal, California sea lion and Weddell seal cDNA.
Genomic DNA was extracted from Cape fur seal liver tissue, subantarctic fur seal mammary gland tissue, ringed seal (Phoca hispida) muscle tissue, Australian sea lion (Neophoca cinerea) muscle tissue, Weddell seal milk and California sea lion milk. The DNA was extracted from tissue and milk samples using previously described methods (Lindquist et al. 1994; Strauss 1998). Genomic α-LA was amplified using cDNA oligonucleotide primers to produce the full length gene. Sequences are deposited in GenBank under accession numbers EF465494–EF465507.
The lactose synthesis assay was performed as previously described (Nagamatsu & Oka 1980) with slight modifications detailed in the electronic supplementary material.
3. Results and discussion
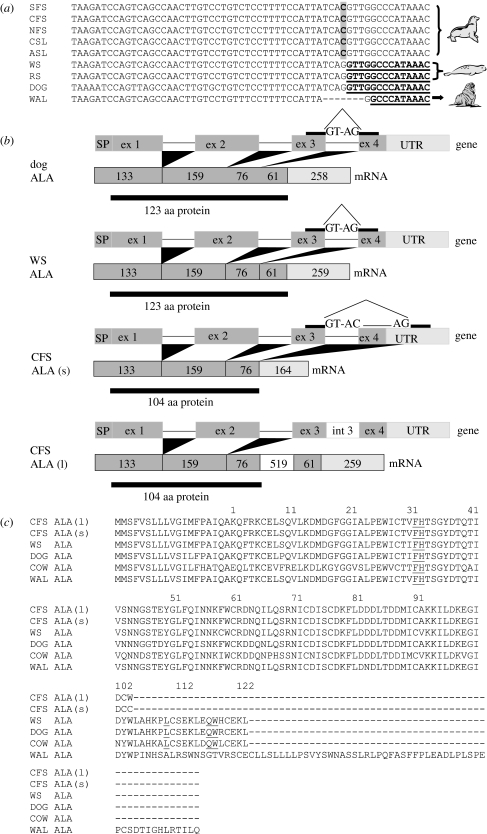
In the present study two alternatively spliced α-LA cDNA variants (545 and 1221 bp) were identified from cDNA isolated from mammary gland tissue of a lactating Cape fur seal. The genomic α-LA sequence from Cape fur seal plus two additional fur seals (northern Callorhinus ursinus and subantarctic) and two sea lions (Australian and California) were cloned and compared with dog genomic α-LA, revealing a consistent base change in the third intron splice site indicating aberrant splicing of the fourth exon (figure 1a). This aberrant splicing is responsible for the two cDNA variants isolated from Cape fur seal mammary tissue, with the longer variant (α-LA(l)) maintaining the third intron and the shorter variant (α-LA(s)) using a splice site further downstream in what is exon 4 in other α-LA homologues (figure 1b). The predicted Cape fur seal α-LA protein sequences for both variants are truncated at 104 residues due to a stop codon introduced into the reading frame and differ only in their C-terminal residue (figure 1c; electronic supplementary material).
Figure 1.
(a) Genomic sequence alignments of intron 3/exon 4 boundary of α-LA from subantarctic (SFS), Cape (CFS) and northern fur seal (NFS), California (CSL) and Australian sea lion (ASL), Weddell (WS) and Ringed seal (RS), domestic dog (NP_001003129) and walrus (WAL). The mutation is bold and shaded in otariids α-LA, and dashes indicate the deletion in walrus α-LA. Exon 4 is underlined and bold. (b) Gene structure of α-LA (ALA) from domestic dog, Weddell seal and the two Cape fur seal variants (CFS (l), long; CFS (s), short). Intron 3 splice sites are shown. (c) Protein alignment of α-LA from Cape fur seal (long and short), Weddell seal, domestic dog (Q9N2G9), cow (P00711) and walrus (SFS and CSL α-LA proteins found to be identical to CFS; electronic supplementary material). Residues essential for lactose synthesis are underlined.
The residues necessary for lactose synthesis activity are Phe31, His32, Leu110, Gln117 and Trp118 of α-LA (Brew 2003). With the exception of the first two, these essential residues are found in exon 4, which is disrupted in otariid α-LA and, hence, would account for the lack of lactose in otariid milks. This was further confirmed by the lack of lactose synthesis activity (pmole mg−1 tissue min−1) in mammary gland tissue from a subantarctic fur seal following addition of exogenous bovine β-GT (36±1, control 41±12), as previously reported for the California sea lion (Johnson et al. 1972), whereas addition of exogenous bovine α-LA resulted in a 10-fold increase in lactose synthesis activity (400±43, control 37±0.2).
Sequencing of Atlantic walrus (Odobenus rosmarus) genomic α-LA revealed a seven base pair deletion where the otariid mutation has occurred (figure 1a). The deletion predicts a frame shift within exon 4 and the predicted protein (176 amino acids) is longer than common α-LA homologues (figure 1c). Since exon 4 is disrupted, walrus α-LA is also predicted to be unable to produce lactose, supporting earlier analyses of walrus milk (Fay 1982). In contrast, analysis of genomic α-LA in two phocid species (Weddell seal and ringed seal) showed that both had the correct nucleotide base in the third intron splice site and were predicted to produce the normal splice variant (figure 1a). To confirm this, we isolated Weddell seal α-LA cDNA (701 bp) and the predicted protein sequence of 123 residues is consistent with most other α-LA homologues (figure 1b,c).
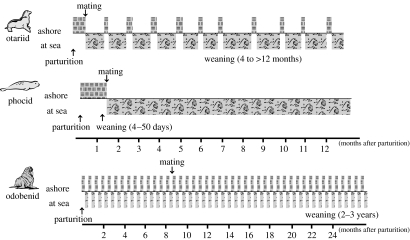
The ancestral pinniped would have moved into environments that required the separation, both spatially and temporally, of the acquisition of resources for milk production and its delivery to offspring (Boyd et al. 1999). In adapting to these environments, phocids became large with relatively short, intense lactation periods (4–50 days depending on species) during which females fast on land (Oftedal et al. 1987). In contrast, otariids adopted longer lactation periods (from 4 to more than 12 months depending on species; Oftedal et al. 1987) during which mothers regularly undertake long foraging trips to sea (more than 23 days in some species; Georges & Guinet 2000), leaving their pup to fast on land (figure 2). While the production of milk during foraging trips is greatly reduced (Arnould & Boyd 1995), the mammary gland remains fully functional (Sharp et al. 2005) and a high rate of milk production is rapidly restored when the female returns to land (Arnould & Boyd 1995). In contrast, phocids cease normal mammary gland function within 24 hours of suckling stimulus removal (Lang et al. 2005). This ability of otariids to maintain mammary gland function despite the extremely long inter-suckling intervals is unique among mammals; normally lack of suckling stimulus rapidly leads to a decline in galactopoietic hormones and accumulation of milk within the gland, with local factors subsequently initiating mammary involution (Green & Streuli 2004).
Figure 2.
Generalized diagram of the different lactation strategies undertaken by the three families of pinnipeds. The otariid and phocid reproductive cycle is generally annual, whereas odobenid lactation can last up to two years with the pup accompanying its mother out to sea.
While the specific trigger for initiating mammary involution is unknown, one suggestion is a mechanoreceptor response to stretching as the mammae distend in the absence of suckling (Green & Streuli 2004). The mutation in the α-LA gene of otariid, resulting in the secretion of milk with low water content due to the absence of lactose, may prevent extensive distension of the mammary gland during long foraging trips to sea and, thus, prevent the occurrence of mammary involution. Indeed, mice created with α-LA deficiency produce highly viscous milk due to the absence of lactose. After 5 days of lactation, while the milk is too viscous to secrete, the tight junctions between the mammary epithelial cells appear to be maintained, indicating that alveoli were not excessively distended as would be expected in wild-type mice after extended milk stasis (Stinnakre et al. 1994).
Additionally, mammary-derived factors, including α-LA, may also initiate involution (Green & Streuli 2004). Human and bovine α-LA can cause apoptosis (Hakansson et al. 1995; Xu et al. 2005). In order to do so, α-LA must cross the lipid bilayer membranes in a molten globule confirmation, where it can disrupt the histones (Mok et al. 2007). The findings of the present study suggest that the truncated α-LA proteins of otariids would not be able to form a stable molten globule conformation (Demarest et al. 2001); therefore, they would not have the same apoptotic effect as that in the mammary glands of other mammals. Thus, the mutation in otariid α-LA could have allowed the maintenance of mammary function, despite the extremely long inter-suckling intervals, through the absence of lactose and/or the absence of α-LA apoptotic ability.
Odobenids have also adopted a strategy of long lactation (more than 1 year), but the mother feeds in close proximity to the pup enabling her to suckle frequently (Oftedal et al. 1987; figure 2). Recent phylogenetic data suggest that otariids and odobenids share a common ancestor that split from the phocid ancestor (Arnason et al. 2006). A probable scenario is that the point mutation in the α-LA splice site occurred first in the common ancestor of otariids and odobenids, followed by the deletion in the odobenids after divergence. The lack of further mutations in α-LA in otariids may lie in its antimicrobial activity (Pellegrini et al. 1999), which would be beneficial to the health of the mammary gland during the long inter-suckling intervals. In contrast, the ability to suckle regularly may have reduced the need for antimicrobial activity in the mammary gland of odobenids and, therefore, selection to maintain α-LA resulting in the second mutation. Consequently, the mutation in the α-LA splice site in the common ancestor of otariids and odobenids could have influenced the divergence in lactation strategies within pinnipeds.
Acknowledgments
The assistance of R. E. Stewart, B. Dickerson, M. A. Hindell, T. D. Williams, R. Mcintosh, K. N. Cane and J. A. Sharp in obtaining milk, tissue, DNA and RNA samples is gratefully acknowledged. We also thank K. N. Cane, E. A. Pharo and K. R. Nicholas for their useful comments. This research was supported by the Geoffrey Gardiner Foundation and the CRC for Innovative Dairy Products. C.M.R. was a recipient of a Dairy Australia Postgraduate Student Award.
Supplementary Material
Lactose synthesis assay; Oligonucleotide Design; Intron 3 splice site; Subantarctic fur seal and California sea lion α-LA cDNAs
References
- Arnason U, Gullberg A, Janke A, Kullberg M, Lehman N, Petrov E.A, Vainola R. Pinniped phylogeny and a new hypothesis for their origin and dispersal. Mol. Phylogenet. Evol. 2006;41:345–354. doi: 10.1016/j.ympev.2006.05.022. doi:10.1016/j.ympev.2006.05.022 [DOI] [PubMed] [Google Scholar]
- Arnould J.P.Y, Boyd I.L. Temporal patterns of milk production in Antarctic fur seals (Arctocephalus gazella) J. Zool. 1995;237:1–12. [Google Scholar]
- Boyd I.L, Lockyer C, Marsh H.D. Reproduction in marine mammals. In: Reynolds J.E, Rommel S.A, editors. Biology of marine mammals. Smithsonian Institute Press; Washington, DC: 1999. pp. 218–286. [Google Scholar]
- Brew K. Alpha-lactalbumin. In: Fox P, McSweeney P, editors. Advanced dairy chemistry. vol. 1. Kluwer Academic/Plenum Publishers; Dordrecht, The Netherlands: 2003. pp. 387–419. [Google Scholar]
- Demarest S.J, Horng J.C, Raleigh D.P. A protein dissection study demonstrates that two specific hydrophobic clusters play a key role in stabilizing the core structure of the molten globule state of human α-lactalbumin. Proteins. 2001;42:237–242. doi:10.1002/1097-0134(20010201)42:2<237::AID-PROT110>3.0.CO;2-B [PubMed] [Google Scholar]
- Fay F.H. Ecology and biology of the Pacific Walrus Odobenus rosmarus divergens. North Am. Fauna. 1982;74:1–279. [Google Scholar]
- Georges J.Y, Guinet C. Maternal care in the subantarctic fur seals on Amsterdam Island. Ecology. 2000;81:295–308. [Google Scholar]
- Green K.A, Streuli C.H. Apoptosis regulation in the mammary gland. Cell. Mol. Life Sci. 2004;61:1867–1883. doi: 10.1007/s00018-004-3366-y. doi:10.1007/s00018-004-3366-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson A, Zhivotovsky B, Orrenius S, Sabharwal H, Svanborg C. Apoptosis induced by a human milk protein. Proc. Natl Acad. Sci. USA. 1995;92:8064–8068. doi: 10.1073/pnas.92.17.8064. doi:10.1073/pnas.92.17.8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.D, Christiansen R.O, Kretchmer N. Lactose synthetase in mammary gland of the California sea lion. Biochem. Biophys. Res. Commun. 1972;47:393–397. doi: 10.1016/0006-291x(72)90726-7. doi:10.1016/0006-291X(72)90726-7 [DOI] [PubMed] [Google Scholar]
- Lang S.L.C, Iverson S.J, Bowen W.D. Individual variation in milk composition over lactation in harbour seals (Phoca vitulina) and the potential consequences of intermittent attendance. Can. J. Zool. 2005;83:1525–1531. doi:10.1139/z05-149 [Google Scholar]
- Lindquist S, Hansson L, Hernell O, Lonnerdal B, Normark J, Stromqvist M, Bergstrom S. Isolation of messenger-RNA and genomic DNA from epithelial-cells in human-milk and amplification by PCR. Biotechniques. 1994;17:692–696. [PubMed] [Google Scholar]
- Mepham T.B. Open University Press; Milton Keynes, UK: 1987. Physiology of lactation. [Google Scholar]
- Mok K.H, Pettersson J, Orrenius S, Svanborg C. HAMLET, protein folding, and tumor cell death. Biochem. Biophys. Res. Commun. 2007;354:1–7. doi: 10.1016/j.bbrc.2006.12.167. doi:10.1016/j.bbrc.2006.12.167 [DOI] [PubMed] [Google Scholar]
- Nagamatsu Y, Oka T. Purification and characterization of mouse α-lactalbumin and preparation of its antibody. Biochem. J. 1980;185:227–237. doi: 10.1042/bj1850227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftedal O.T, Boness D.T, Tedman R.A. The behaviour, physiology, and anatomy of lactation in the pinnipedia. Curr. Mammal. 1987;1:175–245. [Google Scholar]
- Pellegrini A, Thomas U, Bramaz N, Hunziker P, von Fellenberg R. Isolation and identification of three bactericidal domains in the bovine α-lactalbumin molecule. Biochim. Biophys. Acta. 1999;1426:439–448. doi: 10.1016/s0304-4165(98)00165-2. doi:10.1016/S0304-4165(98)00165-2 [DOI] [PubMed] [Google Scholar]
- Pilson M.E.Q. Absence of lactose from the milk of the Otarioidea, a superfamily of marine mammals. Am. Soc. Zool. 1965;5:220–221. [Google Scholar]
- Sharp J.A, Cane K, Arnould J.P, Nicholas K.R. The lactation cycle of the fur seal. J. Dairy Res. 2005;72:81–89. doi: 10.1017/s0022029905001251. doi:10.1017/S0022029905001251 [DOI] [PubMed] [Google Scholar]
- Stinnakre M.G, Vilotte J.L, Soulier S, Mercier J.C. Creation and phenotypic analysis of α-lactalbumin-deficient mice. Proc. Natl Acad. Sci. USA. 1994;91:6544–6548. doi: 10.1073/pnas.91.14.6544. doi:10.1073/pnas.91.14.6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss W.M. Preparation of genomic DNA from mammalian tissue. In: Ausubel F.M, Brent R, Kingston R.E, Moore D.D, Seidman J.G, Smith J.A, Struhl K, editors. Current protocols in molecular biology. Wiley; New York, NY: 1998. pp. 2.2.1–2.2.3, unit 2.2. [Google Scholar]
- Urashima T, Arita M, Yoshida M, Nakamura T, Arai I, Saito T, Arnould J.P, Kovacs K.M, Lydersen C. Chemical characterisation of the oligosaccharides in hooded seal (Cystophora cristata) and Australian fur seal (Arctocephalus pusillus doriferus) milk. Comp. Biochem. Physiol. B. 2001;128:307–323. doi: 10.1016/s1096-4959(00)00327-4. doi:10.1016/S1096-4959(00)00327-4 [DOI] [PubMed] [Google Scholar]
- Xu M, Sugiura Y, Nagaoka S, Kanamaru Y. IEC-6 intestinal cell death induced by bovine milk alpha-lactalbumin. Biosci. Biotechnol. Biochem. 2005;69:1082–1089. doi: 10.1271/bbb.69.1082. doi:10.1271/bbb.69.1082 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lactose synthesis assay; Oligonucleotide Design; Intron 3 splice site; Subantarctic fur seal and California sea lion α-LA cDNAs