Abstract
Humpback whales (Megaptera novaeangliae) exhibit a variety of foraging behaviours, but neither they nor any baleen whale are known to produce broadband clicks in association with feeding, as do many odontocetes. We recorded underwater behaviour of humpback whales in a northwest Atlantic feeding area using suction-cup attached, multi-sensor, acoustic tags (DTAGs). Here we describe the first recordings of click production associated with underwater lunges from baleen whales. Recordings of over 34 000 ‘megapclicks’ from two whales indicated relatively low received levels at the tag (between 143 and 154 dB re 1 μPa pp), most energy below 2 kHz, and interclick intervals often decreasing towards the end of click trains to form a buzz. All clicks were recorded during night-time hours. Sharp body rolls also occurred at the end of click bouts containing buzzes, suggesting feeding events. This acoustic behaviour seems to form part of a night-time feeding tactic for humpbacks and also expands the known acoustic repertoire of baleen whales in general.
Keywords: acoustic behaviour, northwest Atlantic, baleen whale, click, DTAG, feeding
1. Introduction
Broadband, pulsed click sounds are produced by many marine mammals. So far, the best known users of clicks are odontocetes that emit echolocation signals during foraging (Au 1993). Outside odontocete taxa, Parks et al. (2005) described broadband, short duration ‘gunshot’ sounds from North Atlantic right whales (Eubalaena glacialis). These sounds (20 Hz–20 kHz, 1–4 pulses in a group, average interpulse interval 35 ms) were associated with surface active (mating) groups or produced by lone males and appear to be used in social communication. Some transient click sounds have also been recorded in the presence of minke whales (Balaenoptera acutorostrata; 4–7 kHz, 7 clicks s−1; Beamish & Mitchell 1973), but without any documentation of concurrent behaviour. One broadband pulse train has been reported from humpbacks (Thompson et al. 1986; 1 ms pulses and irregular interpulse interval), which was attributed to baleen rattle. However, the use of broadband clicks in association with foraging has not been documented for any baleen whale or pinniped, and arguments have been made against echolocation in both of these taxonomic groups (Schusterman et al. 2000; Au et al. 2001).
Humpback whales (Megaptera novaeangliae) are an acoustically active species, known to produce and use various types of sound, including complex songs on their breeding grounds (Payne & McVay 1971; Darling et al. 2006) and cooperative feeding calls in Alaska (D'Vincent et al. 1985). Foraging is confined primarily to higher latitudes, such as our study area, during the summer months. Animals feed by lunging through schools of small fish and euphausiids, often using bubbles or other tactics to corral prey (Hain et al. 1982). However, knowledge about humpback sound production on the feeding grounds is limited, and most comes from the North Pacific (Thompson et al. 1986).
To investigate acoustic and underwater foraging behaviour of North Atlantic humpback whales, we used DTAGs, which are suction-cup tags that record sound and body orientation of tagged individuals (Johnson & Tyack 2003). Since their inception, these tags have been used with a variety of cetaceans and are opening a window into marine mammal behaviour under water that has historically been almost inaccessible. We deployed DTAGs on nine humpback whales in the Gulf of Maine. Of these nine, two individuals displayed a previously unreported acoustic behaviour: multiple bouts of broadband clicks that we termed ‘megapclicks’ (after the scientific name for humpback whales and to distinguish them from the acoustically different odontocete clicks). Here we describe the temporal and spectral patterns of these megapclicks and place them in the context of concurrent behaviours contained in the DTAG record.
2. Material and methods
(a) Data collection
DTAGs (non-invasive, digital, acoustic recording tags with orientation and depth sensors; acoustic sampling rate 64 kHz; sensor sampling rate 50 Hz; Johnson & Tyack 2003) were deployed on feeding humpback whales. Five whales in 2004 and four whales in 2005 were tagged from a 7 m rigid-hulled inflatable boat (RHIB) deployed from a NOAA Research Vessel, the Nancy Foster. Tags were attached via suction cups and were placed midway between the dorsal fin and the blowhole using a 15 m cantilevered pole mounted in the bow of the RHIB (from photographic estimates, the tag on mn178a was approx. 1.5 m further forward than the tag on mn177a). Tagged whales were tracked visually and by VHF radio, with the observation vessel remaining 100 m or further from the tagged animal at all times. Tags remained attached for 21.3 (mn177a) and 23 (mn178a) hours. Both whales were visually observed lunge feeding near the surface after tagging, and continued this behaviour at least until dark.
Clicks were noted when listening to the tag recordings and were later located automatically using custom programs in Matlab. A representative click was bandpass filtered (400–3500 Hz), Hilbert transformed, and cross-correlated with similarly transformed audio files to identify bouts of click sounds. Complete bouts were then extracted manually, and aurally categorized according to the presence or absence of one or more fast-clicking ‘buzzes’ (described below). Presence of buzzes was noted without knowledge of the whale's depth or body orientation. Received levels were calculated after highpass filtering acoustic files at 400 Hz to reduce flow noise and downsampling to an 8 kHz sampling rate to minimize file size. Data were also visualized in GeoZui4D, which integrates time, three-dimensional body orientation and sound (Arsenault et al. 2004). GeoZui4D allowed detailed visualizations of whale behaviour concurrent with megapclick production (video 1, in the electronic supplementary material).
All clicks described here were recorded from a position that is probably off-axis and so may give a poor estimate of the click spectrum and amplitude in the forward direction. No megapclick echoes (from which on-axis properties could have been obtained) were observed using techniques from Johnson et al. (2004). We assumed that the clicks recorded were produced by the tagged whale based on the tight relationship of clicks with body movements (see below), and also the consistency in acoustic characteristics of the clicks. If the recorded clicks were produced by other animals, they would probably have arrived from different directions, and the received levels and spectral information would be more variable across the dataset.
3. Results
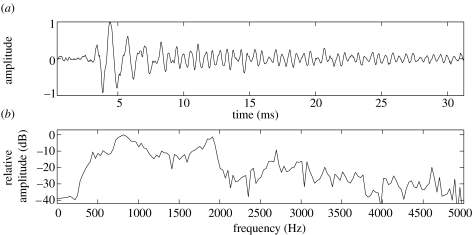
Megapclicks were short pulses of broadband sound (figure 1), grouped together into bouts. A total of 101 bouts (17 from mn177a and 84 from mn178a), containing 34 026 clicks, were identified from two whales on different days. The sounds had peak frequencies of approximately 800 and 1700 Hz (figure 1), and received levels at the DTAG of 143+/−5 dB re 1 μPa pp for mn177a and 154+/−5 dB re 1 μPa pp for mn178a.
Figure 1.
(a) Waveform of a representative megapclick and (b) its normalized spectrum, as recorded by the tag attached midway between the dorsal fin and blowhole. The signal was highpass filtered at 400 Hz to remove flow noise.
(a) Timing
During click bouts, 98% of interclick intervals (ICIs) were between 19 and 200 ms. The shortest ICIs generally occurred at the end of bouts as part of an acceleration in click rate. This pattern was aurally similar to an odontocete or bat ‘terminal buzz’ (e.g. Griffin et al. 1960; Johnson et al. 2004). We therefore used the term ‘buzz’ to describe these sequences, recognizing that they may not serve the same purpose as those recorded from odontocetes or bats. Buzzes contained megapclicks with ICIs of 25 ms or less and had durations of at least 0.5 s. Four click bouts with one or more buzzes were noted from mn177a and 35 from mn178a.
All megapclick bouts occurred during night-time hours (between sunset at 20 : 26 and sunrise at 05 : 05). Megapclick bouts generally occurred near the bottom of dives, and average whale depth at time of bout production was 38 m+/−12 m, which was near the sea floor in this area.
(b) Body orientation
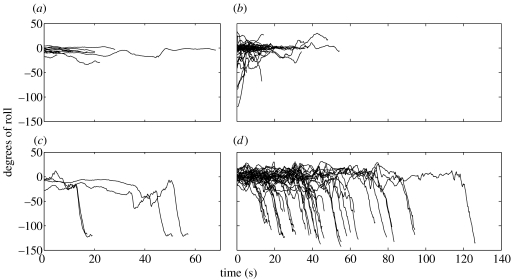
All bouts that ended in buzzes also ended with a sharp body roll by the tagged animal (in both whales, n=39). Almost none (1 of 62) of the bouts lacking buzzes ended with such a roll (figure 2). These rolls were qualitatively similar to body movements during surface feeding lunges. Mean body roll orientations at the end of megapclick bouts with buzzes were −119°+/−2° (mn177a) and −108°+/−22° (mn178a), and when buzzes were not present were −8°+/−7° (mn177a) and −6°+/−16° (mn178a) (figure 2). Rolls with magnitudes and angular rates similar to those co-occurring with buzzes occurred frequently during tag attachments overall (180 performed by mn177a and 515 by mn178a), including some during night-time hours, which is not unusual if these rolls do represent feeding events.
Figure 2.
Degrees of roll from horizontal of tagged animals for the duration of each megapclick bout. (a,b) Whales 177a (n=13) and 178a (n=49), bouts with no terminal buzz. (c,d) Whales 177a (n=4) and 178a (n=35), bouts with terminal buzz(es).
4. Discussion
Few studies of baleen whales have paired the production of specific sounds with specific behaviour, especially under water. Here we have reported production of click sequences by two tagged humpback whales on a feeding ground. These megapclicks were produced during night-time hours and with a pattern of decreased ICIs towards the end of the bouts. These intervals of faster clicking, or buzzes, co-occurred with rolls resembling body movements during feeding lunges. We therefore suggest a foraging function for megapclicks and discuss several hypotheses below.
Given the superficial similarity of the production pattern of megapclicks to odontocete echolocation sounds, biosonar arises as a possible explanation. That humpback whales may echolocate has been proposed in the past, but based on song rather than clicks (Frazer & Mercado 2000). Acoustically, megapclicks recorded here were substantially lower amplitude and frequency and longer duration than odontocete echolocation signals, even given humpbacks' larger body size and tag placement behind the head. These are unlikely properties for signals with an echolocation function. However, the temporal pattern of many of the bouts was reminiscent of odontocete clicks in echolocation-mediated foraging, where terminal buzzes after click bouts appear to be associated with prey-capture attempts (e.g. sperm whale ‘creaks’ in Miller et al. 2004). Depending on hearing sensitivities and directionality of the humpback sound production system, sounds with these characteristics could be useful for some form of rough acoustic detection such as identifying the seafloor or other large target.
Alternatively, megapclicks could be used to manipulate prey. Humpbacks have been shown to manipulate prey in non-acoustic ways (Hain et al. 1982), and some have hypothesized that sound itself may be a means of stunning the prey (Norris & Mohl 1983; but see Benoit-Bird et al. 2006). In the case of humpbacks feeding on bottom-dwelling species (Hain et al. 1995), the whales might use the sounds to flush prey into the water column, as has been speculated for bottlenose dolphins (Nowacek 2005). However, of note is that, of nine animals tagged, only these two have produced clicks, and these are also the only two whales tagged on Jeffreys Ledge, which is an area thought to be dominated by Atlantic herring (Clupea harengus); other tagged whales were located in the Great South Channel and on Platt's Bank and Georges Bank, which may differ in prey composition (Chase 2002).
Many unknowns remain surrounding baleen whale hearing and sound production mechanisms, and this lack of knowledge hinders our interpretation of the function of these megapclicks. Additional recordings of baleen whale clicks and information on how the sounds appear in the far-field are needed to distinguish among various hypotheses for how megapclicks might be used. But, despite uncertainties in function, this is the first documentation of click production by a baleen whale that is probably related to feeding. This discovery furthers our understanding of humpback whale behaviour on the feeding grounds, and also greatly expands the known repertoire of sound production by baleen whales in general.
Acknowledgments
Research was conducted under NMFS permit no. 981-1707-00 and in accordance with animal care and use guidelines in the United States.We thank three anonymous reviewers as well as numerous field volunteers and colleagues, especially C. Ware, T. Hurst, A. Shorter, K. Sardi, M. Weinrich, B. Branstetter, W. Koeppen and E. M. Nosal. Funding provided by the National Oceanic and Atmospheric Administration's National Marine Sanctuary Program and the University of Hawai'i Sea Grant College Program. This is publication no. UNIHI-SEAGRANT-JC-07-02 and no. HIMB 1284.
Supplementary Material
This video, created in GeoZui4D, integrates multiple datasets including acoustics, pitch, roll, heading, and depth. A track was created using swim speed and ocean current approximations, and anchored by GPS locations taken during surface follows of the tagged whale. This representation was used for data visualization and exploration
References
- Arsenault R, Ware C, Plumlee M, Martin S, Whitcomb L, Wiley D, Gross T, Bilgili A. A system for visualizing time varying oceanographic 3D data. Oceans. 2004;2:743–747. doi:10.1109/OCEANS.2004.1405535 [Google Scholar]
- Au W.W.L. Springer; New York, NY: 1993. The sonar of dolphins. [Google Scholar]
- Au W.W.L, Frankel A, Helweg D.A, Cato D.H. Against the humpback whale sonar hypothesis. IEEE J. Oceanic Eng. 2001;26:295–300. doi:10.1109/48.922795 [Google Scholar]
- Beamish P, Mitchell E. Short pulse length audio frequency sounds recorded in the presence of a minke whale, Balaenoptera acutorostrata. Deep Sea Res. 1973;20:375–386. [Google Scholar]
- Benoit-Bird K.J, Au W.W.L, Kastelein R. Testing the odontocete acoustic prey debilitation hypothesis: no stunning results. J. Acoust. Soc. Am. 2006;120:1118–1123. doi: 10.1121/1.2211508. doi:10.1121/1.2211508 [DOI] [PubMed] [Google Scholar]
- Chase B.C. Differences in diet of Atlantic bluefin tuna (Thunnus thynnus) at five seasonal feeding grounds on the New England continental shelf. Fish. Bull. 2002;100:168–180. [Google Scholar]
- Darling J.D, Jones M.E, Nicklin C.P. Humpback whale songs: do they organize males during the breeding season? Behaviour. 2006;143:1051–1101. doi:10.1163/156853906778607381 [Google Scholar]
- D'Vincent C.G, Nilson R.M, Hanna R.E. Vocalization and coordinated feeding behavior of the humpback whale in southeastern Alaska. Sci. Rep. Cet. Res. Tokyo. 1985;36:41–47. [Google Scholar]
- Frazer L.N, Mercado E. A sonar model for humpback whale song. IEEE J. Oceanic Eng. 2000;25:160–182. doi:10.1109/48.820748 [Google Scholar]
- Griffin D.R, Webster F.A, Michael C.R. The echolocation of flying insects by bats. Anim. Behav. 1960;8:141–154. doi:10.1016/0003-3472(60)90022-1 [Google Scholar]
- Hain J.W, Carter G.R, Kraus S.D, Mayo C.A, Winn H.E. Feeding behavior of the humpback whale Megaptera novaeangliae, in the western North Atlantic. Fish. Bull. 1982;80:259–268. [Google Scholar]
- Hain J.W, Ellis S.L, Kenney R.D, Clapham P.J, Gray B.K, Weinrich M.T, Babb I.G. Apparent bottom feeding by humpback whales on Stellwagen Bank. Mar. Mammal Sci. 1995;11:464–479. doi:10.1111/j.1748-7692.1995.tb00670.x [Google Scholar]
- Johnson M.P, Tyack P.L. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Oceanic Eng. 2003;28:3–12. doi:10.1109/JOE.2002.808212 [Google Scholar]
- Johnson M, Madsen P.T, Zimmer W.M.X, Aguilar de Soto N, Tyack P.T. Beaked whales echolocate on prey. Proc. R. Soc. B. 2004;271(Suppl.):S383–S386. doi: 10.1098/rsbl.2004.0208. doi:10.1098/rsbl.2004.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P.J.O, Johnson M.P, Tyack P.L. Sperm whale behaviour indicates the use of echolocation click buzzes ‘creaks’ in prey capture. Proc. R. Soc. B. 2004;271:2239–2247. doi: 10.1098/rspb.2004.2863. doi:10.1098/rspb.2004.2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K.S, Mohl B. Can odontocetes debilitate prey with sound? Am. Nat. 1983;122:85–104. doi:10.1086/284120 [Google Scholar]
- Nowacek D.P. Acoustic ecology of foraging bottlenose dolphins (Tursiops truncatus), habitat-specific use of three sound types. Mar. Mammal Sci. 2005;21:587–602. doi:10.1111/j.1748-7692.2005.tb01253.x [Google Scholar]
- Parks S.E, Hamilton P.K, Kraus S.D, Tyack P.L. The gunshot sound produced by male North Atlantic right whales (Eubalaena glacialis) and its potential function in reproductive advertisement. Mar. Mammal Sci. 2005;21:458–475. doi:10.1111/j.1748-7692.2005.tb01244.x [Google Scholar]
- Payne R, McVay S. Songs of humpback whales. Science. 1971;173:585–597. doi: 10.1126/science.173.3997.585. doi:10.1126/science.173.3997.585 [DOI] [PubMed] [Google Scholar]
- Schusterman R.J, Kastak D, Levenson D.H, Reichmuth C.J, Southall B.L. Why pinnipeds don't echolocate. J. Acoust. Soc. Am. 2000;107:2256–2264. doi: 10.1121/1.428506. doi:10.1121/1.428506 [DOI] [PubMed] [Google Scholar]
- Thompson P.O, Cummings W.C, Ha S.J. Sounds, source levels, and associated behavior of humpback whales, Southeast Alaska. J. Acoust. Soc. Am. 1986;80:735–740. doi: 10.1121/1.393947. doi:10.1121/1.393947 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video, created in GeoZui4D, integrates multiple datasets including acoustics, pitch, roll, heading, and depth. A track was created using swim speed and ocean current approximations, and anchored by GPS locations taken during surface follows of the tagged whale. This representation was used for data visualization and exploration