Abstract
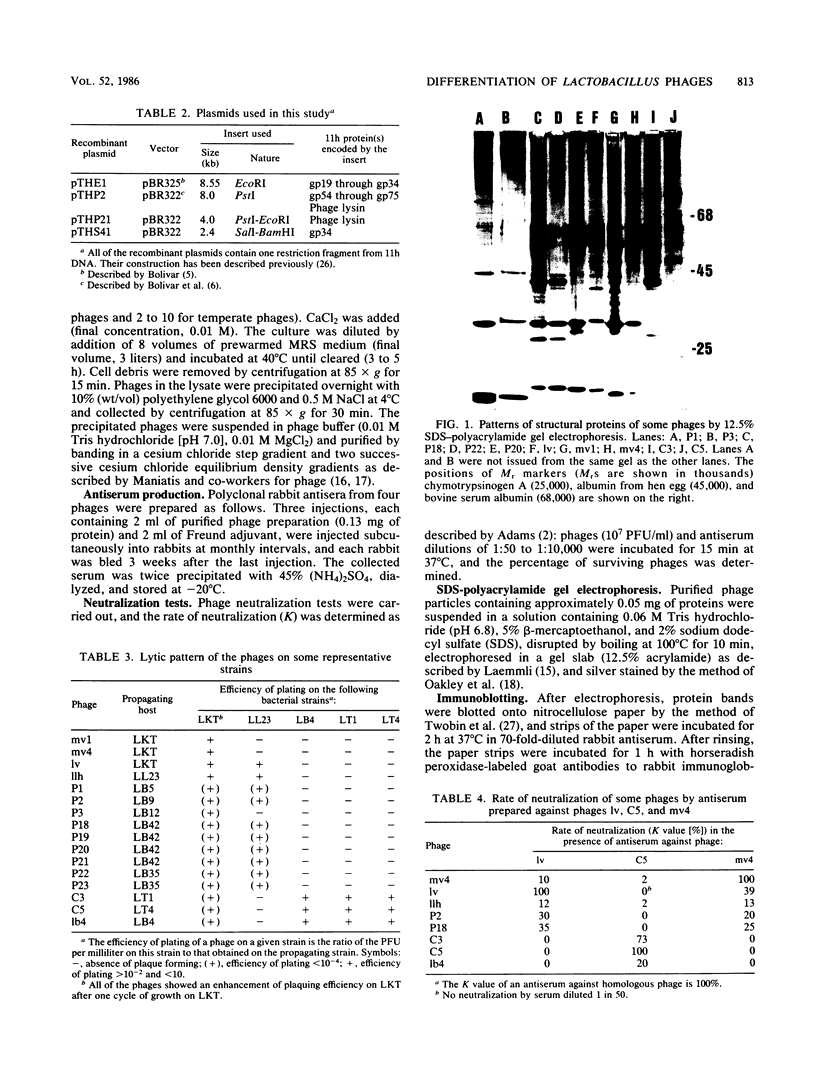
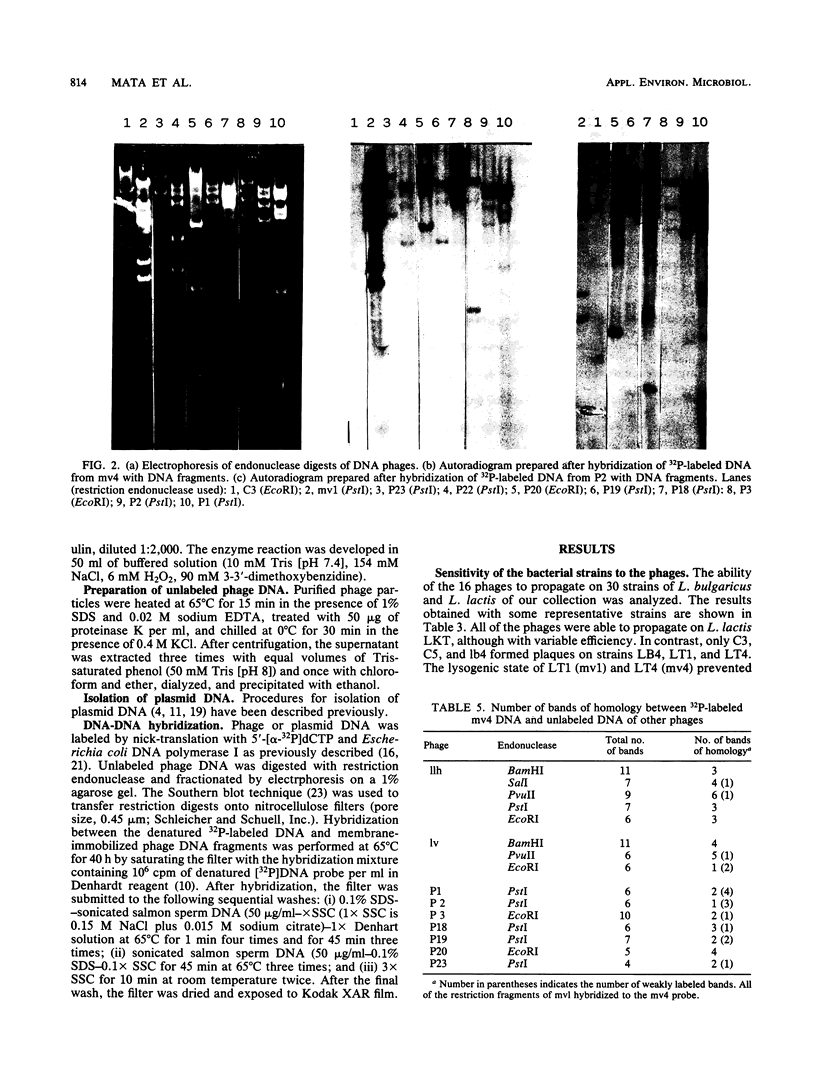
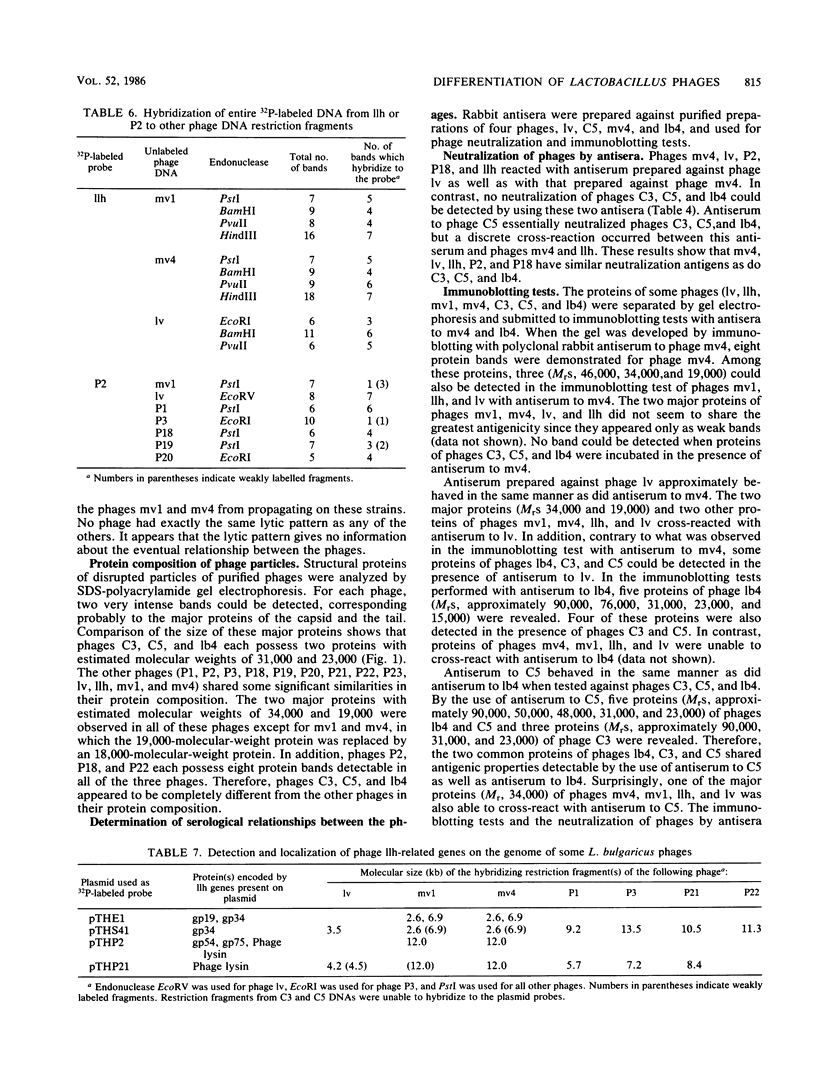
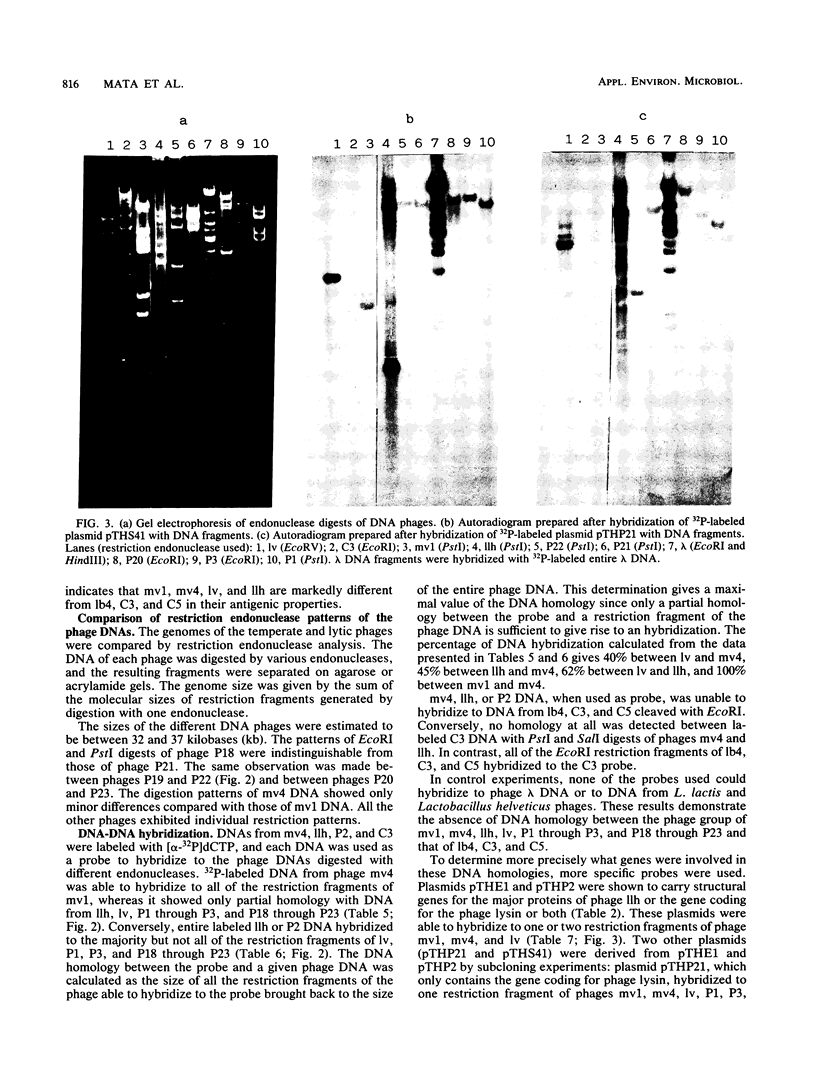
Thirteen virulent phages and two temperate phages of two closely related bacterial species (Lactobacillus lactis and L. bulgaricus) were compared for their protein composition, their antigenic properties, their restriction endonuclease patterns, and their DNA homology. The immunoblotting studies and the DNA-DNA hybridizations showed that the phages could be differentiated into two groups. One group contained 2 temperate phages of L. bulgaricus and 11 virulent phages of L. lactis. Inside each group, at least two common proteins of identical sizes could be detected for each phage. These proteins were able to cross-react in immunoblotting experiments with an antiserum raised against one phage of the same group. Temperate phage DNAs showed partial homology with DNAs from some virulent phages. These homologies seem to be located on the region coding for the structural proteins since recombinant plasmids coding for one of the major phage proteins of one phage were able to hybridize with the DNAs from phages of the same group. These results suggest that temperate and virulent phages may be related to one another.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chopin M. C., Chopin A., Roux C. Definition of bacteriophage groups according to their lytic action on mesophilic lactic streptococci. Appl Environ Microbiol. 1976 Dec;32(6):741–746. doi: 10.1128/aem.32.6.741-746.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis A. W. Differentiation of lactic streptococcal phages into phage species by DNA-DNA homology. Appl Environ Microbiol. 1984 Feb;47(2):343–349. doi: 10.1128/aem.47.2.343-349.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyrolle J., Chopin M. C., Letellier F., Novel G. Lysogenic strains of lactic Acid streptococci and lytic spectra of their temperate bacteriophages. Appl Environ Microbiol. 1982 Feb;43(2):349–356. doi: 10.1128/aem.43.2.349-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Sakurai T., Tsuchida N. Prophage Origin of a Virulent Phage Appearing on Fermentations of Lactobacillus casei S-1. Appl Environ Microbiol. 1983 Feb;45(2):669–674. doi: 10.1128/aem.45.2.669-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sozzi T., Watanabe K., Stetter K., Smiley M. Bacteriophages of the genus Lactobacillus. Intervirology. 1981;16(3):129–135. doi: 10.1159/000149259. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwetter A., Ritzenthaler P., Alatossava T., Mata-Gilsinger M. Physical and genetic characterization of the genome of Lactobacillus lactis bacteriophage LL-H. J Virol. 1986 Sep;59(3):551–555. doi: 10.1128/jvi.59.3.551-555.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]





