Abstract
Tropical forests contain the majority of extant plant diversity and their role as a cradle and/or museum of biodiversity is an important issue in our attempts to assess the long-term consequences of global climate change for terrestrial biomes. Highly diverse groups of liverworts are an often ignored but extremely common element in rainforests, and thus their evolution may shed light on the ecological robustness of rainforest biomes to climate fluctuations. We record a remarkable constant accumulation of diversity through time for the most species-rich family of liverworts, Lejeuneaceae, inferred by divergence time estimates. The observed pattern supports the recently developed concept of a dual role of the tropics as both a museum and a cradle of biodiversity.
Keywords: divergence time estimates, Lejeuneaceae, liverworts
1. Introduction
The current biodiversity crisis, especially in relation to the array of anthropogenic threats to the highly diverse tropical forests, has enhanced our general interest in the role of the tropics in the maintenance and recovery of biodiversity (Pimm et al. 1995; Laurance 2007). Detailed knowledge about these processes may enable us to develop models to predict the response of biodiversity to global warming (Jablonski et al. 2006; Marshall 2006). The tropics are often interpreted as either museums or cradles of biodiversity (Stebbins 1974), but their contribution may be more dynamic. A dual function as both a museum and a cradle of biodiversity may be the more suitable scenario for most organisms (Jablonski et al. 2006; Marshall 2006; McKenna & Farell 2006).
Traditionally, the inference of macroevolutionary patterns relied mainly on the fossil record to infer the origin and extinction of lineages through time. This restricted such studies to organisms with a good fossil record and excluded lineages with poor to nearly absent fossil records, such as many land plant lineages. Liverworts are occasionally well preserved as amber inclusions, but adequate conditions for their preservation in the fossil record have occurred only sporadically in time and space (Grolle & Meister 2004). Recent advancements in bioinformatics and molecular biology now allow us to explore the macroevolution of these lineages using DNA sequence data to estimate divergence times (Kumar 2005). Divergence time estimates are widely used to explore macroevolutionary patterns and processes, e.g. the coinciding diversification of ferns and angiosperms (Schneider et al. 2004), and global patterns of major animal and plant lineages (Brady et al. 2006; Danforth et al. 2006; Hughes et al. 2006; Moreau et al. 2006; Roelants et al. 2007). These studies offer important new insights, which are not possible using the fossil record alone.
These achievements motivated us to investigate the role of the tropics in the sustainability of biodiversity under the pressure of global climate change. Recent studies reported divergent interpretations of the high tropical diversity including evidence for faster evolution (Wright et al. 2006) and gradual accumulation of diversity (Bramley et al. 2004; McKenna & Farell 2006). Here, we infer the diversification pattern of a plant lineage with an arbitrarily distributed fossil record. Lejeuneaceae is the most species-rich family of liverworts and not only forms a particularly important component of the cryptogamic flora of tropical lowland forests, but also contributes substantially to the temperate liverwort flora (Gradstein 1993). Hence these liverworts are ideal candidates for inferring the origin of tropical diversity and their contribution to the non-tropical diversity. Recent studies have revealed their relationship to other liverworts as well as the relationships among the majority of genera within Lejeuneaceae (Wilson et al. 2007). Divergence time estimates have also shown that Lejeuneaceae started to diversify no earlier than the Mid-Cretaceous (Newton et al. 2006; Heinrichs et al. 2007); therefore, they are a further example of a seed-free land plant lineage diversifying in the shadow of angiosperms (Schneider et al. 2004). The biology of these taxa also provides an exceptional opportunity to compare the museum with the cradle hypothesis. These liverworts are generally good long-distance dispersers and their diversification pattern is probably little influenced by local-scale events (Schuster 1983). This renders them more capable of colonizing new habitats in times of climate change, where regions with a previously cooler climate become warmer.
2. Material and methods
The dataset comprising 135 species and four genomic regions (rbcL/psbA/trnL-F/nrITS; 3773 aligned nucleotides) was generated and handled as described in our published exhaustive phylogenetic study on Lejeuneaceae (Wilson et al. 2007). Divergence time estimates were calculated employing a penalized likelihood approach using phylogenetic hypotheses generated in a Bayesian inference of phylogeny as described by Schneider et al. (2004). Lineage-through-time plots were generated for the whole Lejeuneaceae clade and major clades for 100 MCMC-based chronograms or the consensus chronogram.
3. Results
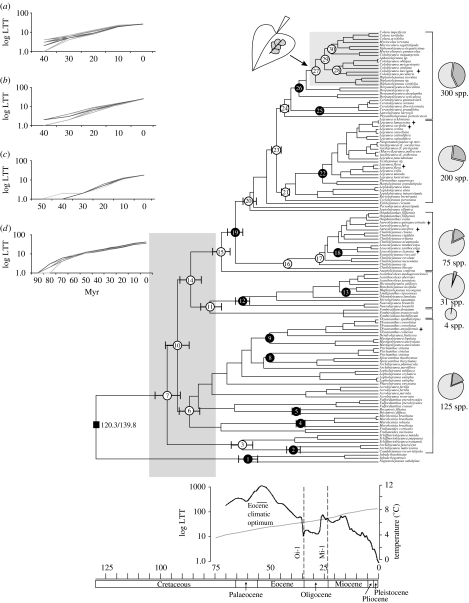
Divergence time estimates provide evidence for a relatively rapid establishment of the major lineages in the Cretaceous (figure 1, nodes 7, 6, 10 and 14), but the majority of observed lineage establishments occurred during the Tertiary. Node 6 (figure 1), in particular, shows a more or less constant rate of separating lineages through the Tertiary. Lineage-through-time plots indicate a nearly constant rate of origin and extinction of lineages during the Tertiary. Net diversification rates do not vary substantially during this period (e.g. node 16, 0.0212; node 21, 0.0215; node 24, 0.0213). This is remarkable since neither the Eocene maximum temperature peak nor the Oligocene glaciations had a strong influence on the rate of origin and extinction of Lejeuneaceae. These patterns were also observed when selected lineages were studied separately. We did not find any evidence for an increased diversification rate for any particular clade, including the derived clade (figure 1, node 24), which includes plants with an epiphyllous habit (growth on living leaves), suggestive of a faster generation time (Gradstein et al. 2006). The taxon sampling, however, is most likely insufficient to infer the rates of clades that diversified after the Late Miocene. This limitation does not apply to the global pattern where a more or less constant rate of diversification was documented. The majority of lineages included in the chronogram occur predominantly to exclusively in the tropics and hence the data support the museum hypothesis. Temperate taxa originated from several independent colonizations as indicated by their distribution in the phylogeny and not from independent radiations into temperate regions. This pattern supports the tropics as the cradle of the current temperate Lejeuneaceae diversity.
Figure 1.
Phylogenetic chronogram for Lejeuneaceae plotted against geological time scale (Gradstein et al. 2004), using mean calibration point age of 130.1 Ma (Heinrichs et al. 2007). Ages for selected nodes (numbered) are given in the electronic supplementary material. Nodes with minimum age fossil constraints are indicated by black circles. Bars at nodes indicate age difference when using minimum and maximum fossil calibration points (120.3/139.8 Ma, respectively). Where no bar is present, difference is negligible. Shaded area at base of tree highlights initial diversification event. Shaded area at node 27 indicates the presence of epiphylly. Pie charts represent the proportion of extant Lejeuneaceae species in each clade. Taxa marked with  indicate temperate species. An averaged record of sea-surface temperatures (Zachos et al. 2001), a proxy for global climate, is presented against the geological time scale. Timing of Miocene (Mi-1) and Oligocene (Oi-1) glaciations and the Eocene climatic optimum are marked on the temperature curve. A mean lineage-through-time (LTT) plot is shown with the temperature curve. LTT plots of 25 randomly selected Bayesian inference trees are shown for nodes (a) 24, (b) 21, (c) 16 and (d) 6.
indicate temperate species. An averaged record of sea-surface temperatures (Zachos et al. 2001), a proxy for global climate, is presented against the geological time scale. Timing of Miocene (Mi-1) and Oligocene (Oi-1) glaciations and the Eocene climatic optimum are marked on the temperature curve. A mean lineage-through-time (LTT) plot is shown with the temperature curve. LTT plots of 25 randomly selected Bayesian inference trees are shown for nodes (a) 24, (b) 21, (c) 16 and (d) 6.
4. Discussion
The rapid divergence of the extant lineages of Lejeuneaceae in the Late Cretaceous is remarkable since the reconstructed phylogeny would indicate a long, static period throughout the Early Cretaceous. In addition, the sister clade of Lejeuneaceae (approx. 750 spp.), the Jubulaceae (approx. 6 spp.), is much less species rich. This pattern may fit a scenario of a rapid rise triggered by the rise of angiosperms (Schuster 1983; Heinrichs et al. 2007), reminiscent of the diversification of derived ferns (Schneider et al. 2004). However, alternative hypotheses involving extinction of ancestral sister lineages in the Late Cretaceous or at the Cretaceous–Tertiary boundary have to be considered. Similar patterns of diversification were documented for birds and mammals (Penny & Philips 2004; Brady et al. 2006). The coincidence of several independent diversifications of unrelated lineages combined with the putative replacement of more ancestral lineages leads to the hypothesis that the Middle and Late Cretaceous was a time of major reorganization of terrestrial habitats and underlying ecological networks (Schneider et al. 2004). Our results add evidence to previous reports of decoupling of diversity accumulation of plants and insects in the Early Tertiary (Wilf et al. 2006).
Observed static net divergence rates throughout the Cenozoic are very much in contrast to the pattern observed in the Cretaceous. Climatic fluctuations, such as the maximum temperature peaks in the Eocene and major global cooling in the Oligocene, appear to have had very limited influence on the net divergence rate in Lejeuneaceae. The lack of correlation with climate change is in contrast with divergence time estimates reported for some plant lineages, where dramatic fluctuations during the Miocene and Pleistocene correlate well with speciation (Won & Renner 2006). However, the net divergence rate is the composite of speciation and extinction rates, and therefore a constant net rate may result from a strong correlation between these two rates. In addition, some clades may have been more diverse in the past but were subsequently replaced by members of sister clades that diversified in later periods of the Cenozoic. The replacement of species by close relatives from the same genera or family where similar ecologies already exist is thought to be a significant contribution to the persistence and stability of plant communities over significant time periods (DiMichele & Philips 1996; Valiente et al. 2006).
In conclusion, the tropics appear to have constantly accumulated diversity within Lejeuneaceae throughout the Cenozoic, as suggested by the museum hypothesis. They are also the cradle for extant temperate Lejeuneaceae species since all temperate species are nested in clades comprising mainly tropical species. This is the first example of land plants demonstrating robustness of diversity in times of major climate fluctuations. The broad ecological range of most Lejeuneaceae species may be the cause for the observed stability. However, as shown by the pattern for the Cretaceous, the diversity of these plants may change drastically if the general structure of tropical terrestrial habitats is substantially modified.
Acknowledgments
This study was supported by DFG grants. We thank R. Grolle for discussions and B. Press, J. Vogel, A. Schmidt and an anonymous reviewer for their comments.
Supplementary Material
Phylogenetic chronogram for Lejeuneaceae plotted against geological timescale using mean calibration point age of 130.1 Myr (ago) (Heinrichs et al. 2007). Node ages are given in table 3 in the electronic supplementary material. Nodes with minimum age fossil constraints are indicated with black circles
Lejeuneaceae fossils. Ages for fossils were taken from the literature; Dominican (S15), Baltic (S16, S22) and Mexican amber (S17)
Voucher information with Genbank accession numbers. Accession numbers in bold are new for this study. Other sequences are from S1, S10, S12, S13, S14
Dates of nodes as indicated in figure 1 in the electronic supplementary material, using minimum calibration point (120.3 Myr (ago)) and maximum calibration point (139.8 Myr (ago)). Calibration points taken as confidence interval from (S10)
References for supplementary material
Data generation and phylogenetic analyses; Divergence time estimates
References
- Brady S.G, Schultz T.R, Fisher B.L, Ward P.S. Evaluating alternative hypotheses for the early evolution and diversification of ants. Proc. Natl Acad. Sci. USA. 2006;103:18 172–18 177. doi: 10.1073/pnas.0605858103. doi:10.1073/pnas.0605858103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramley G.L.C, Pennington R.T, Zakaria R, Tjitrosoedirdjo S.S, Cronk Q.C.B. Assembly of tropical plant diversity on a local scale: Cyrtandra (Gesneriaceae) on Mount Kerinci Sumatra. Biol. J. Linn. Soc. 2004;81:49–62. doi:10.1111/j.1095-8312.2004.00283.x [Google Scholar]
- Danforth B.N, Sipes S, Fang J, Brady S.G. The history of early bee diversification based on five genes plus morphology. Proc. Natl Acad. Sci. USA. 2006;103:15 118–15 123. doi: 10.1073/pnas.0604033103. doi:10.1073/pnas.0604033103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMichele W.A, Philips T.L. Clades, ecological amplitudes, and ecomorphs: phylogenetic effects and persistence of primitive plant communities in the Pennsylvanian-age tropical wetlands. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996;127:83–105. doi:10.1016/S0031-0182(96)00089-2 [Google Scholar]
- Gradstein S.R. In: Biodiversity and conservation of neotropical montane forests. Churchill S.P, Balslev H, Forero E, Luteyn J.L, editors. The New York Botanical Garden; New York, NY: 1993. p. 21. [Google Scholar]
- Gradstein F, Ogg J, Smith A. Cambridge University Press; Cambridge, UK: 2004. A geologic time scale. [Google Scholar]
- Gradstein S.R, Wilson R, Ilkiu-Borges A.L, Heinrichs J. Phylogenetic relationships and neotenic evolution of Metzgeriopsis (Lejeuneaceae) based on chloroplast DNA sequences and morphology. Bot. J. Linn. Soc. 2006;151:293–308. doi:10.1111/j.1095-8339.2006.00531.x [Google Scholar]
- Grolle R, Meister K. Weissdorn; Jena, Germany: 2004. The liverworts in baltic and bitterfeld amber. [Google Scholar]
- Heinrichs J, Hentschel J, Wilson R, Feldberg K, Schneider H. Evolution of leafy liverworts (Jungermanniidae Marchantiophyta): estimating divergence times from chloroplast DNA sequences using penalized likelihood with integrated fossil evidence. Taxon. 2007;56:31–44. [Google Scholar]
- Hughes C, Eastwood R. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc. Natl Acad. Sci. USA. 2006;103:10 334–10 339. doi: 10.1073/pnas.0601928103. doi:10.1073/pnas.0601928103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski D, Roy K, Valentine J.W. Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science. 2006;314:102–106. doi: 10.1126/science.1130880. doi:10.1126/science.1130880 [DOI] [PubMed] [Google Scholar]
- Kumar S. Molecular clocks: four decades of evolutions. Nat. Rev. Genet. 2005;6:654–662. doi: 10.1038/nrg1659. doi:10.1038/nrg1659 [DOI] [PubMed] [Google Scholar]
- Laurance W.F. Have we overstated the tropical biodiversity crisis? Trends Ecol. Evol. 2007;22:65–70. doi: 10.1016/j.tree.2006.09.014. doi:10.1016/j.tree.2006.09.014 [DOI] [PubMed] [Google Scholar]
- Marshall C.R. Fossil record reveals tropics as cradle and museum. Science. 2006;313:66–67. doi: 10.1126/science.1133351. doi:10.1126/science.1133351 [DOI] [PubMed] [Google Scholar]
- McKenna D.D, Farell B.D. Tropical forests are both evolutionary cradles and museums of leaf beetle diversity. Proc. Natl Acad. Sci. USA. 2006;103:10 947–10 951. doi: 10.1073/pnas.0602712103. doi:10.1073/pnas.0602712103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C.S, Bell C.D, Vila R, Archibald S.B, Pierce N.E. Phylogeny of the ants: diversification in the age of the angiosperms. Science. 2006;312:101–104. doi: 10.1126/science.1124891. doi:10.1126/science.1124891 [DOI] [PubMed] [Google Scholar]
- Newton A, et al. In: Pleurocarpous mosses: systematics and evolution. Newton A.E, Tangney R.S, editors. Taylor and Francis; London, UK: 2006. p. 329. [Google Scholar]
- Penny D, Philips M.J. The rise of birds and mammals: are microevolutionary processes sufficient for macroevolution. Trends Ecol. Evol. 2004;19:516–522. doi: 10.1016/j.tree.2004.07.015. doi:10.1016/j.tree.2004.07.015 [DOI] [PubMed] [Google Scholar]
- Pimm S.L, Russell G.J, Gittleman J.L, Brooks T.M. The future of biodiversity. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. doi:10.1126/science.269.5222.347 [DOI] [PubMed] [Google Scholar]
- Roelants K, Gower D.J, Wilkinson M, Loader S.P, Biju S.D, Guillaume K, Moriau L, Bossuyt F. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA. 2007;104:887–892. doi: 10.1073/pnas.0608378104. doi:10.1073/pnas.0608378104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Schuettpelz E, Pryer K.M, Cranfill R, Magallon S, Lupia R. Ferns diversified in the shadow of angiosperms. Nature. 2004;428:553–557. doi: 10.1038/nature02361. doi:10.1038/nature02361 [DOI] [PubMed] [Google Scholar]
- Schuster R.M. New manual of bryology. vol. 1. Hattori Botanical Laboratory; Nichinan, Japan: 1983. p. 463. [Google Scholar]
- Stebbins G.L. Belknap; Cambridge, MA: 1974. Flowering plants: evolution above the species level. [Google Scholar]
- Valiente Banuet A, Vital Rumebe A, Verdú M, Callaway R.M. Modern quaternary plant lineages promote diversity through facilitation of ancient Tertiary linages. Proc. Natl Acad. Sci. USA. 2006;103:16 812–16 817. doi: 10.1073/pnas.0604933103. doi:10.1073/pnas.0604933103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilf P, Labandeira C.C, Johnson K.R, Ellis B. Decoupled plant and insect diversity after the End-Cretaceous extinction. Science. 2006;313:1112–1115. doi: 10.1126/science.1129569. doi:10.1126/science.1129569 [DOI] [PubMed] [Google Scholar]
- Wilson R, Gradstein S.R, Schneider H, Heinrichs J. Unravelling the phylogeny of Lejeuneaceae (Jungermanniopsida) Mol. Phylogenet. Evol. 2007;43:270–282. doi: 10.1016/j.ympev.2006.10.017. doi:10.1016/j.ympev.2006.10.017 [DOI] [PubMed] [Google Scholar]
- Won H, Renner S.S. Dating dispersal and radiation in the gymnosperm Gnetum (Gnetales)—clock calibration when outgroup relationships are uncertain. Syst. Biol. 2006;55:610–622. doi: 10.1080/10635150600812619. doi:10.1080/10635150600812619 [DOI] [PubMed] [Google Scholar]
- Wright S, Keeling J, Gillman L. The road from Santa Rosalia: a faster tempo of evolution in tropical climates. Proc. Natl Acad. Sci. USA. 2006;103:7718–7722. doi: 10.1073/pnas.0510383103. doi:10.1073/pnas.0510383103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. doi:10.1126/science.1059412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic chronogram for Lejeuneaceae plotted against geological timescale using mean calibration point age of 130.1 Myr (ago) (Heinrichs et al. 2007). Node ages are given in table 3 in the electronic supplementary material. Nodes with minimum age fossil constraints are indicated with black circles
Lejeuneaceae fossils. Ages for fossils were taken from the literature; Dominican (S15), Baltic (S16, S22) and Mexican amber (S17)
Voucher information with Genbank accession numbers. Accession numbers in bold are new for this study. Other sequences are from S1, S10, S12, S13, S14
Dates of nodes as indicated in figure 1 in the electronic supplementary material, using minimum calibration point (120.3 Myr (ago)) and maximum calibration point (139.8 Myr (ago)). Calibration points taken as confidence interval from (S10)
References for supplementary material
Data generation and phylogenetic analyses; Divergence time estimates