Abstract
The role of temperature is central to both organic evolution and ecological processes. However, how temperature affects selection on body size is unknown. We tested whether small seed beetles (Stator limbatus) have an advantage over large beetles during scramble competition for mates, and whether this advantage varies with temperature. Within lines of beetles artificially selected to be large versus small, small males have a significant advantage over large males in scramble competition for females because the former takeoff more quickly and thus reach females before larger males. Selection favouring small male body size is significantly (and substantially) more intense at cooler temperatures. The adaptive significance of small male body size thus depends on ambient temperature.
Keywords: thermal adaptation, small body size advantage, sexual selection, scramble competition, sexual size dimorphism, insect flight
1. Introduction
Most studies of selection on body size demonstrate that large individuals have substantial fitness advantages over small animals. Mechanisms producing selection for small body size are poorly understood (Blanckenhorn 2000, 2005), other than the fitness costs of extended development, where animals take longer to mature, increasing generation time and thus reducing fitness. Although large males are typically favoured in contest competition, small males may have an advantage in scramble competition (Ghiselin 1974; Steele & Partridge 1988; Reiss 1989; Blanckenhorn et al. 1995; Moya-Laraño et al. 2002; Crompton et al. 2003).
Temperature affects almost all traits of organisms, and thus has strong direct and indirect effects on fitness (Atkinson 1994; Clarke 2003). Though temperature effects on development (and thus phenotypic plasticity) are well understood, few studies have examined how temperature affects sexual and natural selection on body size and whether this selection affects males differently from females. Since scramble competition is dependent on male movement and locomotion is temperature dependent in ectotherms, variation in temperature probably affects selection on male size via scramble competition (Willmer 1991). However, to our knowledge, there is little evidence for this hypothesis.
The seed beetle Stator limbatus (Coleoptera, Chrysomelidae and Bruchinae) is unusual among insects in that males are larger than females despite an absence of male–male contest competition (Savalli & Fox 1998). Female mate choice and fecundity selection via nuptial gifts in male ejaculates favour large males (Savalli & Fox 1998; Moya-Laraño & Fox 2006). We used S. limbatus males that vary substantially in adult body size to test whether selection on body size occurs during scramble competition (when males ‘race’ to find females) and whether the magnitude of this selection varies with temperature.
2. Material and methods
(a) Scramble competition experiment
We used populations of S. limbatus that had been selected for large and small body size via artificial selection (electronic supplementary material). Each line was tested at high (30°C) and low (20°C) temperatures mimicking warm and cold days in the population of origin (http://www.wrcc.dri.edu/). Each of the eight lines (electronic supplementary material) was tested at two temperatures yielding 16 trials. In each trial, we released simultaneously virgin males from one of the selection lines (sample sizes in figure 1) at one end of a 4×4 m room. An equal number of females were released from a cage at the opposite side of the room at a window (20 cm height×50 cm wide) located 3 m away from the male release point. Preliminary observations showed that, as are most insects, these beetles are attracted by the light. To ensure directional flight towards females, we thus released females on the target source of natural light. All beetles were placed in the room in cages 30 min prior to release to allow acclimation to the trial temperature, after which we opened both cages and observed the females for 30 min. We recorded (i) all males arriving at the females and (ii) all males successfully mating with a female. The 30 min period was enough to allow most males to fly to the females, since most beetles arrived within the first 15 min. Beetles were weighed to the nearest 0.1 mg before each trial. We then weighed all recaptured males.
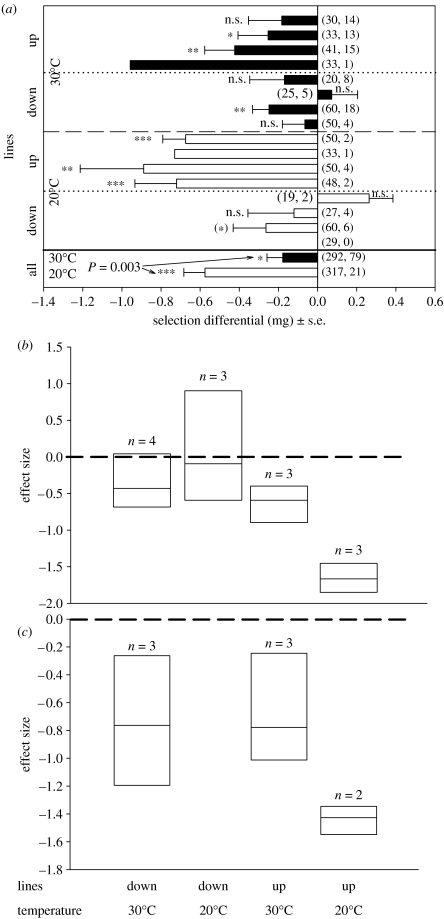
Figure 1.
(a) Selection differentials for body size of males successfully reaching the female area. A negative value means selection against large male body size within a selection line. The y-axis indicates the lines used (selection for large or small beetles) and temperature for each trial (see electronic supplementary material for an explanation of the selection lines). Numbers between parentheses denote the number of males released and recaptured in each trial. ***p<0.001, **p<0.01, *p<0.05, (*) p<0.1. The bottom part shows temperature-dependent selection when beetles from all lines have been pooled in a single analysis (the p-value denotes significant differences between temperatures). (b,c) Meta-analytical selection intensities (hedges d+; with lower and upper bias 95% CIs) on body size calculated across artificial selection lines. Selection calculated defining successful males as those that (b) reached the female area or (c) successfully mated. The horizontal dashed line indicates zero selection. A negative effect indicates selection favouring small male body size. Non-overlapping CIs between treatments indicate significant differences. Numbers on top of bars indicate the number of independent selection lines used. The missing box belongs to a group in which not enough beetles were observed mating for meta-analysis.
We calculated directional selection differentials for each line as , where is the average mass of beetles (in milligrams) released and is the average mass of those either (i) captured at the screen with females (i.e. after selection) or more specifically (ii) mating with females. Stabilizing selection differentials were also calculated but none were significant (not shown). We also calculated overall selection differentials within each temperature by pooling the beetles for all the lines and then tested for differences in selection between temperatures by means of a Wald test (Allison 1995). Since selection estimates have high standard errors (binomial errors on small samples), we did not test for differences in S between temperatures for each line. Instead, we ran meta-analyses (Rosenberg et al. 2000) across lines within temperatures (up and down lines at 20°C; up and down lines at 30°C). Effect sizes calculated in meta-analysis (d) parallel standardized selection differentials (the selection differentials divided by the standard deviation of the trait before selection; i.e. selection intensities), corrected for sample sizes. Although meta-analysis has been developed and used mostly to compare two independent samples, one can use the technique to compare paired samples (Dunlop et al. 1996). We then calculated a combined mean effect size (d+) for each of the four groups. Non-overlapping 95% CIs between groups indicate significant differences.
(b) Takeoff experiment
To test whether the pattern of selection in the scramble competition experiments were due to differences in takeoff, we released individual males from the up and down lines at high (30°C, n=216) and low (20°C, n=287) temperature inside a chamber (80 cm long×55 cm wide×40 cm tall). We recorded the timing of takeoff over 5 min. Several beetles did not take off within that time interval and these are treated as censored data. We compared the timing of takeoff between groups by means of survival analysis, which allows the inclusion of censored data. We then used a survival regression analysis to calculate the relationship between body mass and the time to takeoff including lines and temperatures as factors (Allison 1995).
3. Results
(a) Scramble competition experiment
Selection favoured small males because these males were more likely to reach a potential mate than were large males (figure 1). When selection differentials were calculated for each line and temperature, 13 out of 15 trials were negative (sign test, Z=2.6; p=0.01). Six of these estimates of selection were significantly negative and only two were positive (but not significant). Selection was not estimable in one trial for which no beetle was recaptured. Similarly, no standard errors for selection differentials could be calculated for two trials in which only one beetle was recaptured. Selection favouring small body size was much stronger at cooler temperatures (Wald test, Χ12=8.7, p=0.003). The meta-analyses revealed that the intensity of selection favouring small males depends on both the direction of selection (up versus down) and temperature. In down lines, in which males are very small, selection favouring small males is weak whereas in up lines, in which males are very large, selection favouring small males is very intense. However, in the up lines, selection on male size is greater at low temperature (figure 1).
(b) Takeoff experiment
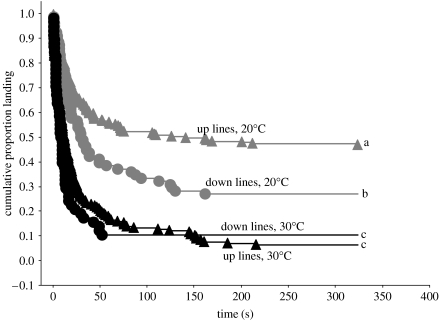
We found that beetles from both lines took off at the same time at high temperature but that smaller males took off sooner at cooler temperatures. A survival analysis comparing the four groups (up and down at high versus low temperature) was highly significant (Χ32=89.4; p<0.0001; figure 2). Beetles from the up lines took off later than beetles from the down lines, but only at lower temperature (log-rank test: Z=2.9, p=0.004 for 20°C; Z=0.8, p=0.420 for 30°C). Mass had a marginally non-significant effect in a survival regression analysis with larger beetles taking off later (β=0.003; Χ12=3.2; p=0.074).
Figure 2.
Kaplan–Meier survival curves for timing of takeoff. Grey lines, low temperature (20°C); black lines, high temperature (30°C); triangles, up lines; circles, down lines. Curves that decline faster indicate faster takeoff. Curves labelled with different letters are significantly different from each other (p<0.01).
4. Discussion
Our results demonstrate that selection via scramble competition favours small males in S. limbatus. This selection opposes other sources of sexual selection that favour large males including fecundity selection and sexual selection (Moya-Laraño & Fox 2006). In addition, the intensity of sexual selection via scramble competition depends on temperature. Although these experiments were conducted in the laboratory, they probably reflect selection patterns in nature because (i) beetles are reared from seeds in nature and must fly to find a mate, (ii) the range of temperatures we used was within the natural range of temperature during the mating season, and (iii) the range of body sizes fits well within the observed natural variation for these beetles (Stillwell et al. in press).
Hypotheses concerning the effect of temperature on flight ability and scramble competition predict an advantage of being large, not small, at lower temperatures because of the better ability of larger animals to retain heat (Willmer 1991). Our results contrast with this; selection for small size was greater at lower than higher temperature, probably reflecting the fact that smaller beetles warm up more quickly due to their higher surface/volume ratio (Atkinson 1994; Dudley 2000). The mechanism for the temperature effect on male flight is not yet known. Both temperature and muscle size affect power output and oscillatory frequency of wing muscles (Harrison & Roberts 2000), and any interaction between the temperature and size effects could generate the observed temperature effects on flight. Faster takeoff could contribute to overall sexual selection on size (Dudley 2000), but other mechanisms (such as differential flying ability) are probably also important. Although S. limbatus is unusual among insects, in that males are larger than females despite an absence of male–male fighting competition, we believe that these mechanisms of selection may apply equally well to insects in which males are smaller than females, since it would explain why males remain smaller than females despite other sources of selection (e.g. contest competition) favouring larger males.
Our results indicate that the effect of body size on scramble competition, and thus selection on body size, is dependent on temperature. Selection on body size should thus vary geographically, especially latitudinal or altitudinally, and may contribute to generate the clines in body size commonly observed in nature (Blanckenhorn et al. 2006). In addition, global warming will affect selection on body size through effects on sexual selection in ectotherms.
Acknowledgments
We thank W. Wallin, L. Hettinger, O. Njoku, L. Hitchcock, A. Lucia DeSouza, D. Johnson, K. Carico and C. Elkins for their help with the laboratory experiments and thank A. Amarillo, C. Stillwell and W. Blanckenhorn for their helpful comments. We thank D. Wise for letting us use his temperature-controlled laboratory room. This work was funded in part by a National Science Foundation grant DEB-02-71929 (to C.W.F.) and a ‘Ramón y Cajal’ research position and grant of the Spanish Ministry of Education and Culture CGL2004-03153 (J.M.L.).
Supplementary Material
Results for the selection experiment on body size in Stator limbatus
References
- Allison P.D. SAS Institute, Inc; Cary, NC: 1995. Survival analysis using the SAS system. [Google Scholar]
- Atkinson D. Temperature and organism size—a biological low for ectotherms? Adv. Ecol. Res. 1994;25:1–58. [Google Scholar]
- Blanckenhorn W.U. The evolution of body size: what keeps organisms small? Q. Rev. Biol. 2000;75:385–407. doi: 10.1086/393620. doi:10.1086/393620 [DOI] [PubMed] [Google Scholar]
- Blanckenhorn W.U. Behavioral causes and consequences of sexual size dimorphism. Ethology. 2005;111:977–1016. doi:10.1111/j.1439-0310.2005.01147.x [Google Scholar]
- Blanckenhorn W.U, Preziosi R.F, Fairbairn D.J. Time and energy constraints and the evolution of sexual size dimorphism—to eat or to mate. Evol. Ecol. 1995;9:369–381. doi:10.1007/BF01237760 [Google Scholar]
- Blanckenhorn W.U, Stillwell R.C, Young K.A, Fox C.W, Ashton K.G. When Rensch meets Bergmann: does sexual size dimorphism change systematically with latitude? Evolution. 2006;60:2004–2011. [PubMed] [Google Scholar]
- Clarke A. Costs and consequences of evolutionary temperature adaptation. Trends Ecol. Evol. 2003;18:573–581. doi:10.1016/j.tree.2003.08.007 [Google Scholar]
- Crompton B, Thomason J.C, McLachlan A. Mating in a viscous universe: the race is to the agile, not to the swift. Proc. R. Soc. B. 2003;270:1991–1995. doi: 10.1098/rspb.2003.2477. doi:10.1098/rspb.2003.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley R. Princeton University Press; Princeton, NJ: 2000. The biomechanics of insect flight: form, function, evolution. [Google Scholar]
- Dunlop W.P, Cortina J.M, Vaslow J.B, Burke M.J. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol. Methods. 1996;1:170–177. doi:10.1037/1082-989X.1.2.170 [Google Scholar]
- Ghiselin M.T. University of California Press; Berkeley, CA: 1974. The economy of nature and the evolution of sex. [Google Scholar]
- Harrison J.F, Roberts S.P. Flight respiration and energetics. Annu. Rev. Physiol. 2000;62:179–205. doi: 10.1146/annurev.physiol.62.1.179. doi:10.1146/annurev.physiol.62.1.179 [DOI] [PubMed] [Google Scholar]
- Moya-Laraño J, Fox C.W. Ejaculate mass, second male size, and moderate polyandry increase female fecundity in a seed beetle. Behav. Ecol. 2006;17:940–946. doi:10.1093/beheco/arl029 [Google Scholar]
- Moya-Laraño J, Halaj J, Wise D.H. Climbing to reach females: Romeo should be small. Evolution. 2002;56:420–425. doi: 10.1111/j.0014-3820.2002.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Reiss M.J. Cambridge University Press; Cambridge, UK: 1989. The allometry of growth and reproduction. [Google Scholar]
- Rosenberg, M. S., Adams, D. C. & Gurevitch, J. 2000 MetaWin: statistical software for meta-analysis, version 2.0. Sunderland, MA: Sinauer Associates.
- Savalli U.M, Fox C.W. Sexual selection and fitness consequences of male body size in the seed beetle Stator limbatus. Anim. Behav. 1998;55:473–483. doi: 10.1006/anbe.1997.0622. doi:10.1006/anbe.1997.0622 [DOI] [PubMed] [Google Scholar]
- Steele R.H, Partridge L. A courtship advantage for small males in Drosophila subobscura. Anim. Behav. 1988;36:1190–1197. doi:10.1016/S0003-3472(88)80078-2 [Google Scholar]
- Stillwell, R. C., Morse, G. E. & Fox, C. W. In press. Geographic variation in body size and sexual size dimorphism of a seed-feeding beetle. Am. Nat. [DOI] [PubMed]
- Willmer P. Thermal biology and mate acquisition in ectotherms. Trends Ecol. Evol. 1991;6:396–399. doi: 10.1016/0169-5347(91)90161-P. doi:10.1016/0169-5347(91)90161-P [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results for the selection experiment on body size in Stator limbatus