Abstract
Our current understanding of the mechanisms that lead to successful biological invasions is limited. Although adaptations play a central role in biological invasions, genetic studies have so far failed to produce a unified theory. The bluespotted cornetfish, a recent Red Sea invader in the Mediterranean Sea via the Suez Canal, provides an ideal case study for research in the mechanisms of invasive genetics. In this study, we show that the invading bluespotted cornetfish underwent a severe population bottleneck that reduced the genetic diversity of this immigrant to only two mitochondrial haplotypes. Although loss of genetic diversity is considered detrimental to the need to adapt to new environments, bluespotted cornetfish experienced an unprecedented success and rapid spread across the Mediterranean.
Keywords: Fistularia commersonii, Lessepsian migration, biological invasions, genetic bottleneck
1. Introduction
The ongoing dispersal of exotic species and the general rearrangement of species geographical distribution are an increasing worldwide phenomenon and currently the most striking biological outcome of global changes (Vitousek et al. 1996). In particular, the recent breaching of biogeographic barriers is allowing the mixing of formerly separate biotas, with unpredictable consequences for ecosystems functioning and evolutionary pathways (Sax et al. 2005).
Following the opening of the Suez Canal in 1869, a migration of marine organisms, termed Lessepsian migrants (after the Canal engineer Ferdinand de Lesseps), started a process of invasion from the Red Sea into the Mediterranean, resulting in ecological impacts on indigenous Mediterranean species. For example, Lessepsian fishes now comprise 65 species (Golani 2006). In general, the specific dynamics of biological invasions are poorly known, yet, in this case, the situation presents some definite advantages for scientists. The date of the opening of the invasion route is known, and the geographical source of the invaders is also known, at least in general terms, as the Red Sea region. Thus, theoretical predictions seemed fairly simple. Some individuals from the Red Sea would enter the Mediterranean via the Suez Canal and would later expand into the wide-open ecological niches. This situation would predict a probable genetic bottleneck due to an invading subsample of the original populations, followed by a fast range expansion, a pattern that is consistent with other documented invasions (Sax et al. 2005). However, in contrast to this prediction, Red Sea and invasive Mediterranean populations displayed a high genetic similarity and no evidence of genetic bottleneck (Golani & Ritte 1999; Bucciarelli et al. 2002; Hassan et al. 2003; Hassan & Bonhomme 2005; Azzurro et al. 2006). Data showed that colonization had occurred by a large number of individuals or by multiple colonization events, or a combination of both. Importantly, all these studies were conducted decades or even more than a century after the invasion of a particular species, thus raising the question of methodological biases.
The situation of the bluespotted cornetfish, Fistularia commersonii, is quite different. This species, which is naturally distributed broadly in the Indo-Pacific, was first recorded in the Mediterranean near Ashdod, Israel in January 2000 (Golani 2000). Since then, it has rapidly spread to the southern shores of Italy (Azzurro et al. 2004) and later to Sardinia (Pais et al. 2007), the furthest a Lessepsian fish migrant has ever been recorded.
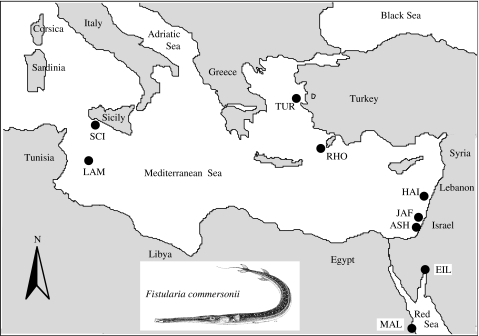
The exact beginning of colonization of a particular Lessepsian migrant is often difficult to determine. However, in the case of a species with a conspicuous external appearance such as F. commersonii, a relatively large and elongated fish (figure 1), it is very likely that they were discovered a very short time after arrival to the new region.
Figure 1.
Mediterranean and Red Sea sampling locations of F. commersonii. Sample codes are given in table 1. Two additional populations, in the Indian Ocean (Seychelles) and the Pacific Ocean (Moorea, French Polynesia), were also sampled. The figure of the bluespotted cornetfish, F. commersonii, is used with the permission of Lucio Valdéz (www.fishars.com).
The goal of this work was to capitalize on the very recent invading event by determining whether this unusually successful invasion followed the original predictions of bottleneck and fast range expansion, without incurring the potential temporal bias described above. We based our study on mitochondrial control region sequences from individuals collected from the Pacific, the Indian Ocean, the Red Sea and populations from the Mediterranean that covered the complete range of invasion.
2. Material and methods
(a) Sample collections and DNA extractions
Samples were collected by spear, hand nets or beach seines in localities described in table 1 and figure 1. Muscle tissue was preserved in 95% ethanol at ambient temperature and DNA was extracted following standard protocols (Sambrook et al. 1989).
Table 1.
Collection localities for Fistularia spp. (Columns represent the number of individuals (n), number of haplotypes (nH), haplotype diversity (HD), date of collection and codes used in figures 1 and 2.)
| species | locality | n | nH | HD | date of collection | code |
|---|---|---|---|---|---|---|
| Fistularia commersonii | ||||||
| natural range | 49 | 46 | 0.997 | |||
| Pacific Ocean | 5 | 5 | 1.000 | |||
| Moorea, French Polynesia | 3 | 3 | 1.000 | Nov 2006 | MOO | |
| Seychelles | 2 | 2 | 1.000 | Jan 2002 | SEY | |
| Red Sea | 44 | 42 | 0.998 | |||
| Marsa Alam, Egypt | 15 | 14 | 0.990 | Dec 2005 | MAL | |
| Eilat, Israel | 29 | 28 | 0.998 | Dec 2004, Jun 2005 | EIL | |
| Mediterranean Sea | 52 | 2 | 0.009 | |||
| Israel | 17 | 1 | 0.000 | |||
| Ashdod | 3 | 1 | 0.000 | Jul 2003 | ASH | |
| Jaffa | 2 | 1 | 0.000 | Oct 2003 | JAF | |
| Haifa | 12 | 1 | 0.000 | Sep 2005 | HAI | |
| Turkey | 1 | 1 | 0.000 | |||
| Karaburnu | 1 | 1 | 0.000 | Sep 2006 | TUR | |
| Greece | 21 | 2 | 0.181 | |||
| Rhodes | 21 | 2 | 0.181 | Sep, Dec 2005 | RHO | |
| Italy | 13 | 2 | 0.530 | |||
| Lampedusa | 12 | 2 | 0.600 | Jan 2005 | LAM | |
| Sciacca | 1 | 1 | 0.000 | Dec 2005 | SCI | |
| outgroups | ||||||
| Fistularia tabacaria | ||||||
| Sao Tome | 1 | Feb 2006 | SAO | |||
| Fistularia petimba | ||||||
| Taiwan | 1 | May 2005 | TAI | |||
(b) PCR amplifications, sequencing and phylogenetic analyses
Amplification of the 5′ hypervariable portion of the mitochondrial control region (also called D-loop) and sequencing followed previously described protocols (Azzurro et al. 2006). Number of haplotypes and haplotype diversity were calculated using the software package DNAsp (Rozas et al. 2003). Phylogenetic relationships were inferred using the neighbour-joining method implemented by PAUP (Swofford 1998), as well as a likelihood Bayesian approach implemented by MrBayes (Huelsenbeck & Ronquist 2001). Statistical confidence in nodes was evaluated using 2000 non-parametric bootstrap replicates (Felsenstein 1985).
3. Results
We sequenced the mitochondrial control region for 103 individuals (52 from the Mediterranean, 44 from the Red Sea, two from the Seychelles and three from French Polynesia, as well as two outgroup species, Fistularia tabacaria from Sao Tomé and Fistularia petimba from Taiwan; table 1).
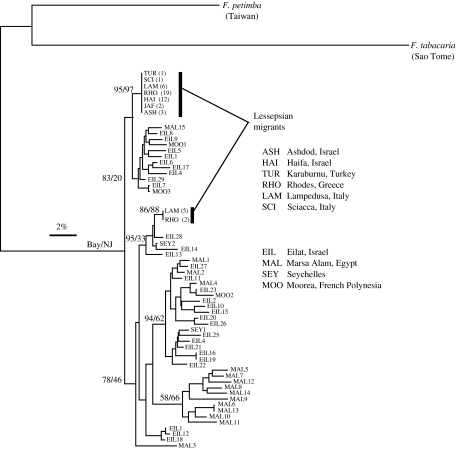
The phylogenetic relationships obtained with the neighbour-joining and Bayesian methods resulted in statistically identical phylogenies, as shown in figure 2. Almost all individuals collected from the natural range of the species were genetically distinct from each other (49 individuals, 46 haplotypes, haplotype diversity 0.997, nucleotide diversity 0.0534; table 1). There was, however, no obvious structure between Red Sea and Indian Ocean/Pacific individuals (figure 2). In contrast, only two haplotypes were observed in the Mediterranean (haplotype diversity 0.009, nucleotide diversity 0.0098). The decrease in genetic diversity between the natural range and the Mediterranean was found to be highly significant (Χ2-test, p<0.001), and it indicates a clear signature of genetic bottleneck and rapid range expansion, which occurred within the past 5 years.
Figure 2.
Phylogenetic relationships of F. commersonii samples based on mitochondrial control region sequences based on Bayesian and neighbour-joining reconstruction methods. Numbers next to the main nodes correspond to Bayesian consensus numbers (left figures) and neighbour-joining bootstrap support (right figures, 2000 replicates). Sample codes and information are provided in the figure and in table 1. The two Mediterranean haplotypes are identified in the figure. Two outgroups were used, F. petimba, collected in Taiwan, and F. tabacaria, collected in Sao Tomé.
The two Mediterranean haplotypes were carried by 45 individuals represented in all populations, and seven individuals from Lampedusa (five) and Rhodes (two). Considering the low frequency of this haplotype, the fact that it was not recovered from other Mediterranean populations was not found to be statistically significant (Χ2, p>0.0.5).
4. Discussion
Golani et al. (2002) claimed that the traverse of the Suez Canal has a strong stochastic component. The detection of only two haplotypes in Mediterranean populations of the bluespotted cornetfish clearly illustrates this point. While F. commersonii took 130 years to cross the length of the Canal, it took only 2 years to disperse westward and reach Italy. The species is now well established in the Mediterranean with the common presence of both juvenile and adult individuals.
Considering the dates of collection and locations of our samples, it seems probable that the Mediterranean populations of bluespotted cornetfish represent a single invasion event by as few as two females. There is no particular reason to think that a disproportionately large number of males would have accompanied that invasion, although nuclear markers, such as microsatellites, would be necessary to settle that question.
Our study underscores a well-known dilemma in invasion biology, where bottlenecked populations with typically low evolutionary potential and perhaps low reproductive fitness can still become successful in their new environment. High migration rates, where repeated introductions occur to overcome low genetic diversity and inbreeding, have been proposed as a potential solution to this dilemma (Frankham 2005), but, apparently, this is not the case for F. commersonii. Our findings reaffirm the difficulty of predicting the potential for invasion success and adaptation of a new invader on the basis of its genetic diversity.
In addition, the rapid dispersal of F. commersonii raises some as yet unanswered questions. Dispersal is likely to be accomplished primarily by larvae, which are known to grow relatively large before settlement (transformation at 60 mm; Watson & Sandknop 1996). Water circulation in the eastern Mediterranean basin is well documented (Hamad et al. 2006) and could explain such rapid dispersal, even if the larval period (which in this case is unknown) is relatively short. Yet, the underlying mechanisms that promoted the dispersal and the successful establishment of this tropical fish remain to be determined. While there is a lack of any recent dramatic temperature increase in the Eastern Mediterranean that could specifically account for this rapid dispersal, other studies point towards the overall warming of the Mediterranean (Perry et al. 2005; Bianchi 2007). Thus, a combination of abiotic and ecological factors, such as high niche opportunities (Shea & Chesson 2002) and/or a particular pre-adaptation, allowed the bluespotted cornetfish to succeed in its new environment.
While previous studies on Lessepsian migrants showed a surprising lack of bottlenecks, the case of F. commersonii provided an opportunity to study the earliest stages of biological invasion in the Mediterranean. As predicted, this invasion resulted from the success of very few individuals. In recent years, more tropical species have successfully invaded the Eastern Mediterranean (Akyol et al. 2005; Bariche & Saad 2005). By closely monitoring their advances, we will be able to replicate this study and determine if the results obtained with the bluespotted cornetfish correspond to a general pattern of Lessepsian invasions.
Acknowledgments
We would like to thank Giuseppe Bucciarelli and Sergio Floeter for providing the F. commersonii sample from Turkey and the F. tabacaria sample from Sao Tomé, respectively.
References
- Akyol O, Unal V, Ceyhan T, Bilecenoglu M. First confirmed record of Lagocephalus sceleratus (Gmelin, 1789) in the Mediterranean Sea. J. Fish Biol. 2005;66:1183–1186. doi:10.1111/j.0022-1112.2005.00667.x [Google Scholar]
- Azzurro E, Pizzicori P, Andaloro F. First record of Fistularia commersonii (Fistularidae) from the central Mediterranean. Cybium. 2004;28:72–74. [Google Scholar]
- Azzurro E, Golani D, Bucciarelli G, Bernardi G. Genetic of the early stage of invasion of the Lessepsian rabbitfish Siganus luridus. J. Exp. Mar. Biol. Ecol. 2006;333:190–201. doi:10.1016/j.jembe.2005.12.002 [Google Scholar]
- Bariche M, Saad M. Settlement of the lessepsian blue-barred parrotfish Scarus ghobban (Teleostei: Scaridae) in the eastern Mediterranean. J. Mar. Biolog. Assoc. 2. Biodiversity records. 2005 www.mba.ac.uk/jmba/pdf/5049.pdf [Google Scholar]
- Bianchi C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiol. 2007;580:7–21. doi:10.1007/s10750-006-0469-5 [Google Scholar]
- Bucciarelli G, Golani D, Bernardi G. Genetic cryptic species as biological invaders: the case of Lessepsian fish migrant, the hardyhead silverside Atherinomorus lacunosus. J. Exp. Mar. Biol. Ecol. 2002;273:143–149. doi:10.1016/S0022-0981(02)00138-7 [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. doi:10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- Frankham R. Invasion biology—resolving the genetic paradox in invasive species. Heredity. 2005;94:385. doi: 10.1038/sj.hdy.6800634. doi:10.1038/sj.hdy.6800634 [DOI] [PubMed] [Google Scholar]
- Golani D. First record of the Bluespotted cornetfish from the Mediterranean. J. Fish Biol. 2000;56:1545–1547. doi:10.1111/j.1095-8649.2000.tb02163.x [Google Scholar]
- Golani D. The Indian scad (Decapterus russelli), (Osteichthyes: Carangidae), a new Indo-Pacific invader of the eastern Mediterranean. Sci. Mar. 2006;70:603–605. [Google Scholar]
- Golani D, Ritte U. Genetic relationship in goatfishes (Mullidae: Perciformes) of the Red Sea and the Mediterranean, with remarks on Suez Canal migrants. Sci. Mar. 1999;63:129–135. [Google Scholar]
- Golani, D., Orsi-Relini, L., Massuti, E. & Quignard, J. P. 2002 CIESM atlas of exotic species in the Mediterranean, vol. 1 (ed. F. Briand). Fishes. Monaco: CIESM Publications.
- Hamad N, Millot C, Taupier-Letage I. The surface circulation in the eastern basin of the Mediterranean Sea. Sci. Mar. 2006;70:457–503. [Google Scholar]
- Hassan M, Bonhomme F. No reduction in neutral variation of mitochondrial and nuclear genes for a Lessepsian migrant, Upeneus moluccensis. J. Fish Biol. 2005;66:865–870. doi:10.1111/j.0022-1112.2005.00643.x [Google Scholar]
- Hassan M, Harmelin-Vivien M, Bonhomme F. Lessepsian invasion without bottleneck: example of two rabbitfish species (Siganus rivulatus and Siganus luridus) J. Exp. Mar. Biol. Ecol. 2003;291:219–232. [Google Scholar]
- Huelsenbeck J.P, Ronquist F. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. doi:10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Pais A, Merella P, Follesa M.C, Garippa G. Westward range expansion of the Lessepsian migrant Fistularia commersonii (Fistulariidae) in the Mediterranean Sea, with notes on its parasites. J. Fish Biol. 2007;70:269–277. doi:10.1111/j.1095-8649.2006.01302.x [Google Scholar]
- Perry A.L, Low P.J, Ellis J.R, Reynolds J.D. Climate change and distribution shifts in marine fishes. Science. 2005;308:1912–1914. doi: 10.1126/science.1111322. doi:10.1126/science.1111322 [DOI] [PubMed] [Google Scholar]
- Rozas J, Sánchez-Del Barrio J.C, Messeguer X, Rozas R. DNAsp, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics. 2003;19:2496–2497. doi: 10.1093/bioinformatics/btg359. doi:10.1093/bioinformatics/btg359 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E.F, Maniatis T. 2nd edn. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. Molecular cloning: a laboratory manual. [Google Scholar]
- Sax D.F, Stachowicz J.J, Gaines S.D. Sinauer Associates; Sunderland, MA: 2005. Species invasions: insights into ecology, evolution, and biogeography; p. 495. [Google Scholar]
- Shea K, Chesson P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002;17:170–176. doi:10.1016/S0169-5347(02)02495-3 [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 1998. PAUP*: phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Vitousek P.M, D'Antonio C.M, Loope L.L, Westbrooks R. Biological invasions as global environmental change. Am. Sci. 1996;84:468–478. [Google Scholar]
- Watson W, Sandknop E.M. Fistulariidae: cornetfishes. In: Moser H.G, editor. The early stages of fishes in the California current region. California Cooperative Oceanic Fisheries Investigations (CalCOFI) atlas no. 33. Allen Press, Inc.; Lawrence, KS: 1996. pp. 718–723 (see also pp. 1505.) [Google Scholar]