Abstract
Sperm competition theory predicts that males should increase their expenditure on the ejaculate with increasing risk of sperm competition, but decrease their expenditure with increasing intensity. There is accumulating evidence for sperm competition theory, based on examinations of testes size and/or the numbers of sperm ejaculated. However, recent studies suggest that ejaculate quality can also be subject to selection by sperm competition. We used experimental manipulations of the risk and intensity of sperm competition in the cricket, Teleogryllus oceanicus. We found that males produced ejaculates with a greater percentage of live sperm when they had encountered a rival male prior to mating. However, when mating with a female that presented a high intensity of sperm competition, males did not respond to risk, but produced ejaculates with a reduced percentage of live sperm. Our data suggest that males exhibit a fine-tuned hierarchy of responses to these cues of sperm competition.
Keywords: strategic ejaculation, ejaculate quality, sperm viability
1. Introduction
Sperm competition has become widely recognized as a potent force of sexual selection, generating behavioural, physiological and morphological adaptations that serve to ensure that the copulating male's sperm are used for fertilization (Simmons 2001). Parker (1970) recognized that there was every reason to expect selection to operate on the ejaculate, because it is the ejaculate that is at the front line in sperm competition. To this end, an extensive game theoretical base has been developed with which to predict the evolutionary effects of sperm competition on male expenditure on the ejaculate (Parker 1998). There is accumulating empirical support for the prediction of this theory that sperm competition can favour increased expenditure on sperm production. Thus, among species from a broad range of taxa, positive evolutionary associations have been found between investment in testes mass and estimates of the strength of selection generated by sperm competition (Harcourt et al. 1995; Birkhead & Møller 1998; Byrne et al. 2002). Within species studies have also found that exposing males to an elevated risk of sperm competition can result in them transferring more sperm at copulation (Wedell et al. 2002; Pound & Gage 2004). However, there are also cases in which researchers have failed to find these predicted associations (e.g. Schaus & Sakaluk 2001; Byrne & Roberts 2004).
Most empirical tests of sperm competition theory have measured expenditure as the numbers of sperm ejaculated. However, recent comparative studies of insects and rodents have shown that sperm competition can have a positive evolutionary association with sperm quality (Hunter & Birkhead 2002; Gomendio et al. 2006). In fishes with alternative male mating tactics, sneaks do not produce more sperm, but produce sperm with greater levels of ATP activity and/or faster swimming speed (Vladic & Järvi 2001). Human males ejaculate fewer sperm with greater motility when exposed to images depicting sperm competition (Kilgallon & Simmons 2005). These studies suggest that sperm quality rather than quantity can be the focus of selection under sperm competition, questioning the current focus on sperm numbers (Snook 2005). A neglect of the importance of sperm quality may underlie the findings of empirical studies that appear counter to sperm competition theory.
We examined phenotypic plasticity in ejaculate quality using the cricket, Teleogryllus oceanicus. In this species, the number of sperm has little impact on a male's competitive fertilization success (Simmons et al. 2003). However, males vary in the viability of their sperm (the proportion of sperm in an ejaculate that are alive), and although sperm viability has little effect on fertilization success when females mate with a single male, it has a strong impact on a male's competitive fertilization success when females mate with more than one male (García-González & Simmons 2005). Thomas & Simmons (2007) found that when males were paired with multiply mated females they produce ejaculates with lower sperm viability than when paired with unmated or single mated females. These data suggest that males adjust the quality of their ejaculates in relation to sperm competition intensity. Here we report the results of an experiment in which we manipulated a male's perception of both sperm competition risk (the probability that a female will mate with more than one male) and intensity (the number of males competing for fertilizations). Importantly, previous sperm competition studies have not considered how these cues interact in influencing male expenditure on the ejaculate, making interpretations of their findings difficult (Engqvist & Reinhold 2005). We find that males exhibit a fine-tuned hierarchy of responses to these cues of sperm competition.
2. Material and methods
Crickets were obtained from a large outbred laboratory stock. Sexes were separated at the final instar. Males were placed into individual containers on the day of their adult moult, and females were held in single or mixed sex containers. Male and female crickets were between 10 and 15 days of adult age when used in experiments, which were conducted during the first 3 hours of the dark phase in a constant-temperature room at 29°C. Males were allocated at random to one of four treatments: high or low sperm competition risk and zero or high sperm competition intensity.
Sperm competition risk and intensity were manipulated through a series of interaction cycles conducted over 3 consecutive days. At the onset of the dark phase, a companion cricket was introduced to each subject male for a period of 60 min, and then removed for 30 min. Subject males experienced a total of three interaction cycles with the same companion cricket. In the high-risk treatment, subjects were provided a companion male, while in the low-risk treatment, subjects were provided with a companion female. For those males introduced to a companion female, mating attempts were interrupted. Immediately following the final interaction cycle, subject males were introduced to a novel female and allowed to mate. Half of the males in each risk treatment were provided with unmated females, while half were provided with females that had been housed with constant access to males prior to experiments, both as interaction partners and as final mating partners. Females with constant access to males mate continuously, and thus presented an immediate high intensity of sperm competition for our subject males. Unmated females presented no immediate sperm competition. Interaction cycles were repeated over 3 consecutive days. Thus, each subject male interacted with one companion male and three female copulation partners, or one companion female and three female copulation partners. Following mating on the third day, subject males were left with their female to produce a second spermatophore. When the male began to court, his spermatophore was removed from the sub-genital pouch and the viability of sperm assayed.
We used the live/Dead sperm viability assay (Molecular Probes). Spermatophores were ruptured in 20 μl of Beadle saline (128.3 mM NaCl, 4.7 mM KCl and 23 mM CaCl2). A 5 μl aliquot of the diluted ejaculate was mixed with 5 μl 1 : 50 diluted 1 mM SYBER-14. The sample was incubated in the dark for 10 min before adding 2 μl propidium iodide and incubating for a further 10 min. Samples were then viewed under a florescence microscope. The number of live (stained green by SYBER-14) and dead (stained red by propidium iodide) sperm in a total of 500 sperm was determined, and the sperm viability calculated as the percentage of live sperm.
3. Results
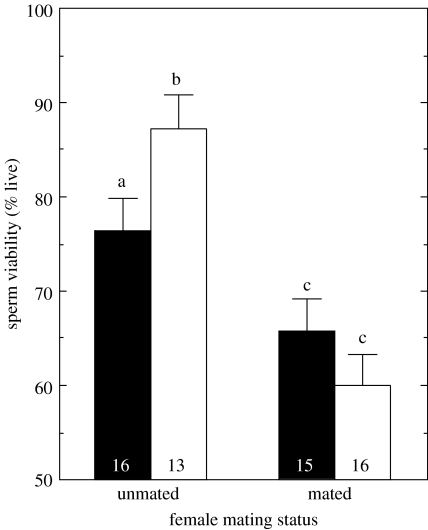
The mean percentage of live sperm in the ejaculate was 71.7±16.6 (N=60). Sperm viability was arcsine square-root transformed to achieve a normal distribution for statistical analysis (Shapiro–Wilk W=0.975, p=0.245). However, to ease interpretation, untransformed means are presented in figure 1. Mating treatment explained 40.6% of the variance in sperm viability (whole model: F3,56=12.76, p<0.0001). There was a significant main effect of sperm competition intensity (multiple mated or unmated female; F1,56=33.44, p<0.0001) and no significant main effect of sperm competition risk (prior interactions with rival males or with females; F1,56=0.75, p=0.391). However, there was a significant interaction between risk and intensity treatments (F1,56=5.70, p=0.020). When males were offered previously mated females, they produced ejaculates with the lowest sperm viability. When offered females that had not mated previously, males produced ejaculates with higher sperm viability following interaction cycles with rival males and lower sperm viability following interaction cycles with females (figure 1).
Figure 1.
Percentage of viable sperm in the ejaculates produced by male crickets, T. oceanicus, in the low (unmated females) and high (multiply mated females) sperm competition intensity, and low (solid bars) and high (open bars) sperm competition risk treatments. Means with different letters differ significantly; sample sizes shown within bars.
4. Discussion
Our data suggest that male T. oceanicus have a fine-grained hierarchy of ejaculate responses to the risk and intensity of sperm competition. Males responded to manipulations of risk when the intensity of sperm competition was zero, but did not respond to risk when the intensity of sperm competition was high. Our finding that males reduced sperm viability with multiple mated females replicates those of Thomas & Simmons (2007), who found, using a repeated measures design, that individual males reduced sperm viability when offered a multiply mated female compared with either an unmated or single mated female. Although we did not assess the number of sperm in the ejaculate in our current experiment, Thomas & Simmons (2007) showed that males did not show phenotypic plasticity in the number of sperm ejaculated.
Engqvist & Reinhold (2005) drew attention to several pitfalls in experiments designed to test predictions from sperm competition theory. For example, it is not clear how manipulations of sex ratio or the number of interacting males might influence a male's perception of sperm competition; the presence of multiple males may influence both the immediate risk and future intensity of sperm competition, and it is unclear how these cues should interact to influence male expenditure. Our experimental manipulations exposed subjects to just one other male or no other males, so that perceptions of risk should have been independent of intensity. Our manipulation of intensity provided a salient cue, intensity was either zero (unmated female) or high (multiply mated female). The cue used by males to assess intensity is most likely to be chemical. Ivy et al. (2005) showed that female crickets transfer olfactory compounds to their mates during copulation, and the same may be true for males. Thus, males may detect chemical cues from rival males on their mates, as shown recently for Drosophila (Friberg 2006). Importantly, our data clearly show how interactions between risk and intensity cues can influence male ejaculation strategies; when intensity is high males pay little attention to risk. Future studies of strategic ejaculation need to consider carefully the multitude of cues that can convey conflicting information regarding risk and/or intensity (Engqvist & Reinhold 2005).
Our study provides just the second case in which males have been shown to strategically adjust ejaculate quality. Kilgallon & Simmons (2005) showed how human males produce ejaculates with greater sperm motility when perceptions of sperm competition were increased. Although we can only speculate on the mechanism by which adjustment is achieved, the most likely candidate may be variation in seminal fluid composition. The seminal fluid of both insects and humans contains a cocktail of compounds, many of which influence the capacitation, motility and nourishment of sperm (Poiani 2006). Males can become depleted of seminal fluid, often when they have adequate sperm remaining (Lefevre & Jonsson 1962), so that prudent allocation of costly seminal fluid components to the ejaculate may maximize male fitness. Indeed, recent theoretical approaches show how non-sperm ejaculate components should respond to risk and intensity of sperm competition, where they influence the competitive weight of sperm (Cameron et al. 2007). Future work will address the proximate mechanisms underlying the observed phenotypic plasticity in ejaculate quality.
Acknowledgments
This work was supported by the Australian Research Council and the School of Animal Biology, University of Western Australia.
References
- Birkhead T.R, Møller A.P. Academic Press; London, UK: 1998. Sperm competition and sexual selection. [Google Scholar]
- Byrne P.G, Roberts J.D. Intrasexual selection and group spawning in quacking frogs (Crinea georgiana) Behav. Ecol. 2004;15:872–882. doi:10.1093/beheco/arh100 [Google Scholar]
- Byrne P.G, Roberts J.D, Simmons L.W. Sperm competition selects for increased testes mass in Australian frogs. J. Evol. Biol. 2002;15:347–355. doi:10.1046/j.1420-9101.2002.00409.x [Google Scholar]
- Cameron E, Day T, Rowe L. Sperm competition and the evolution of ejaculate composition. Am. Nat. 2007;169:E158–E172. doi: 10.1086/516718. doi:10.1086/516718 [DOI] [PubMed] [Google Scholar]
- Engqvist L, Reinhold K. Pitfalls in experiments testing predictions from sperm competition theory. J. Evol. Biol. 2005;18:116–123. doi: 10.1111/j.1420-9101.2004.00792.x. doi:10.1111/j.1420-9101.2004.00792.x [DOI] [PubMed] [Google Scholar]
- Friberg U. Male perception of female mating status: its effect on copulation duration, sperm defence and female fitness. Anim. Behav. 2006;72:1259–1268. doi:10.1016/j.anbehav.2006.03.021 [Google Scholar]
- García-González F, Simmons L.W. Sperm viability matters in insect sperm competition. Curr. Biol. 2005;15:271–275. doi: 10.1016/j.cub.2005.01.032. doi:10.1016/j.cub.2005.01.032 [DOI] [PubMed] [Google Scholar]
- Gomendio M, Martin-Coello J, Crespo C, Magaña C, Roldan E.R.S. Sperm competition enhances functional capacity of mammalian spermatozoa. Proc. Natl Acad. Sci. USA. 2006;103:15 113–15 117. doi: 10.1073/pnas.0605795103. doi:10.1073/pnas.0605795103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt A.H, Purvis A, Liles L. Sperm competition: mating system, not breeding season, affects testes size of primates. Funct. Ecol. 1995;9:468–476. doi:10.2307/2390011 [Google Scholar]
- Hunter F.M, Birkhead T.R. Sperm viability and sperm competition in insects. Curr. Biol. 2002;12:121–123. doi: 10.1016/s0960-9822(01)00647-9. doi:10.1016/S0960-9822(01)00647-9 [DOI] [PubMed] [Google Scholar]
- Ivy T.M, Weddle C.B, Sakaluk S.K. Females use self-referent cues to avoid mating with previous mates. Proc. R. Soc. B. 2005;272:2475–2478. doi: 10.1098/rspb.2005.3222. doi:10.1098/rspb.2005.3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgallon S.J, Simmons L.W. Image content influences mens semen quality. Biol. Lett. 2005;1:235–255. doi: 10.1098/rsbl.2005.0324. doi:10.1098/rsbl.2005.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre G.J, Jonsson U.B. Sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics. 1962;47:1719–1736. doi: 10.1093/genetics/47.12.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Parker G.A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London, UK: 1998. pp. 3–54. [Google Scholar]
- Poiani A. Complexity of seminal fluid: a review. Behav. Ecol. Sociobiol. 2006;60:289–310. doi:10.1007/s00265-006-0178-0 [Google Scholar]
- Pound N, Gage M.J.G. Prudent sperm allocation in Norway rats, Rattus norvegicus: a mammalian model of adaptive ejaculate adjusment. Anim. Behav. 2004;68:819–823. doi:10.1016/j.anbehav.2004.02.004 [Google Scholar]
- Schaus J.M, Sakaluk S.K. Ejaculate expenditures of male crickets in response to varying risk and intensity of sperm competition: not all species play games. Behav. Ecol. 2001;12:740–745. doi:10.1093/beheco/12.6.740 [Google Scholar]
- Simmons L.W. Princeton University Press; Princeton, NJ: 2001. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Simmons L.W, Wernham J, García-González F, Kamien D. Variation in paternity in the field cricket Teleogryllus oceanicus: no detectable influence of sperm numbers or sperm length. Behav. Ecol. 2003;14:539–545. doi:10.1093/beheco/arg038 [Google Scholar]
- Snook R.R. Sperm in competition: not playing by the numbers. Trends. Ecol. Evol. 2005;20:46–53. doi: 10.1016/j.tree.2004.10.011. doi:10.1016/j.tree.2004.10.011 [DOI] [PubMed] [Google Scholar]
- Thomas M.L, Simmons L.W. Male crickets adjust the viability of their sperm in response to female mating status. Am. Nat. 2007;170:190–195. doi: 10.1086/519404. doi:10.1086/519404 [DOI] [PubMed] [Google Scholar]
- Vladic T.V, Järvi T. Sperm quality in the alternative reproductive tactics of Atlantic salmon: the importance of the loaded raffle mechanism. Proc. R. Soc. B. 2001;268:2375–2381. doi: 10.1098/rspb.2001.1768. doi:10.1098/rspb.2001.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedell N, Gage M.J.G, Parker G.A. Sperm competition, male prudence and sperm-limited females. Trends. Ecol. Evol. 2002;17:313–320. doi:10.1016/S0169-5347(02)02533-8 [Google Scholar]