Abstract
In Drosophila melanogaster, accessory gland proteins (Acps) that a male transfers during mating affect his reproductive success by altering the female's behaviour and physiology. To test the role of male condition in the expression of Acps, we manipulated the pre-adult environment and examined adult males for relative transcript abundance of nine Acps, and for post-copulatory traits that Acps influence. Larval culture density had no effect on any measured trait. Larval nutrient availability impacted the number of sperm transferred and stored, the male's ability to induce refractoriness in his mate, but relative transcript abundance of only a single Acp (Acp36DE). Reduced male body size due to low yeast levels affected sperm competition. Our data indicate that some female-mediated post-copulatory traits (induced refractoriness and sperm transfer and storage) might be influenced by the male's developmental environment, but relative expression of most Acps and some traits they influence (P1′) are not.
Keywords: accessory gland proteins, sperm competition, sperm storage, transcript levels, body size
1. Introduction
In Drosophila melanogaster, accessory gland proteins (Acps) that males transfer to females during mating impact traits associated with post-copulatory sexual selection by modulating the behaviour and physiology of mated females. Acps regulate egg laying and oviposition, affect sperm storage, reduce a female's receptivity to remating and associate with variation in sperm competition phenotypes (reviewed in Wolfner 2002; Kubli 2003; Wolfner et al. 2005; Wong & Wolfner 2006).
If Acps are more costly to produce than other non-sexually selected traits, we predicted that they may exhibit a relative reduction in expression under stress (Andersson 1994). Such condition dependence could explain the maintenance of genetic variation in these important traits (Rowe & Houle 1996). Since larval nutrient availability and culture density are known to affect the adult pool of available resources (Mueller et al. 1993), we explored whether the transcript levels of nine Acp genes (Acp29AB, Acp32CD, Acp33A, Acp36DE, Acp53Ea, CG8137, CG17331, CG31872 and ovulin) measured in adult males vary in response to larval rearing environment. We also examined whether traits that Acps influence (sperm competition, female refractoriness to remating, sperm transfer and storage) could also be affected.
2. Material and methods
(a) Rearing conditions
Flies were reared in 25×95 mm vials containing 10 ml of media without the addition of dry yeast. Control flies were reared as 20 larvae/vial on yeast/dextrose medium (1220 ml distilled water, 12 g agar, 100 g dextrose, 100 g brewer's yeast, 0.5 ml of 8.3% phosphoric acid and 0.5 ml of 83.6% propionic acid). To alter the nutrient availability, yeast or dextrose was reduced to 50 or 10%. Density was manipulated on control medium to either 90 or 210 larvae per vial. Environmental manipulations were similar to those used in other Drosophila studies (e.g. Amitin & Pitnick 2006).
Flies were from a wild-type, inbred Canton-S stock maintained in half-pint bottles on yeast/dextrose medium at 25°C under a 12 : 12 hour light : dark cycle for several generations. Twenty arbitrarily chosen females were allowed to oviposit overnight on 35×10 mm Petri dishes containing grape juice medium (2714 ml H2O, 2275 ml grape juice, 110 g agar, 290 g dextrose, 115 g sucrose, 90 g yeast, 110 ml of 1.25 M NaOH, 28 ml of 8.3% phosphoric acid and 28 ml of 83.6% propionic acid). First instar larvae were used to set up treatments. Newly eclosed virgin males were collected over CO2 and maintained in single-sex vials on control medium (approx. 20 flies per vial) for 3 or 4 days prior to measuring phenotypes.
(b) Relative Acp transcript abundance
Three-day-old virgin males were frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using Trizol (Invitrogen), DNase-treated (Roche) and cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen) primed with oligo dT(16). Six extractions were conducted for each condition and two replicate quantitative reverse-transcription PCRs (qRT-PCR) were completed for each cDNA extraction.
Relative Acp transcript abundance was measured using qRT-PCR on an ABI Prism 7000 according to the manufacturer's protocols for SYBR Green. Detailed methods and primer sequences (except for Acp29AB, whose primers were TGGAGTTTAAGGCCCAGATG (forward) and GCCATTTTCAACCAGTTTGG (reverse)) are described in Fiumera et al. (2005). To control for body size and efficiencies of RNA extraction and cDNA synthesis, we regressed each Acp's inverse critical threshold value (1/CT) from the qRT-PCR against its corresponding ‘1/CT’ measure for an internal control gene, RPL32. Analysis of variance (ANOVA) (using regression residuals) was used to test for treatment effects with sequential Bonferroni corrections (Rice 1989). Using raw CT values provided similar results (data not shown).
(c) Sperm competition phenotypes
Only the ‘defense’ components of sperm competitive ability were measured, because the proportion of offspring sired by cn bw marker males when they were the second male to mate with a doubly mated female (P2′) was nearly 100% across different larval environments in preliminary experiments (data not shown). Refractoriness is the proportion of females mated to experimental or control males that do not remate with a tester male. P1′ is the proportion of offspring sired by the experimental or control males when they are the first males to mate with a doubly mated female. Fecundity is the total number of offspring produced by each doubly mated female. Productivity was assessed by singly mating each female to males from the yeast treatments only and tallying progeny over 14 days. Approximately 30 females were used for each treatment.
One-way ANOVA was used to test for an effect of the larval environment on P1′ (arcsine-square-root transformed), female fecundity (square root transformed) and productivity (squared). Permutation tests based on chi-squared statistics (Matlab) were used to test for significant heterogeneity among treatments in female refractoriness. Sequential Bonferroni corrections (Rice 1989) were applied.
(d) Sperm transfer and storage
Three-day-old virgin marker females (cn bw) were mated to three- or four-day-old males reared on 100, 50 or 10% yeast and frozen in liquid nitrogen immediately after copulation (to estimate sperm transferred) or after 8 hours (to estimate sperm stored) and stored at −80°C. Uteri (for sperm transfer) and seminal receptacles (for stored sperm) were dissected in 50% acetic acid and incubated in orcein stain (2% orcein and 0.25% carmine dissolved in 60% acetic acid) for 1 hour and then mounted on a slide containing a drop of acetic acid, covered with a cover slip and sealed with nail polish. Sperm were counted under 100× magnification light microscopy. Data were analysed using ANOVA.
(e) Body size
Four-day-old male flies were dried at 60°C for 24 hours in an oven and weighed on a Sartorius microbalance. We used linear regression to examine effects of body size on treatments. For P1′, refractoriness, sperm transfer and storage, we compared treatment means of body weight versus treatment means of each trait and used the residuals in ANOVA if r2 was significant (p≤0.05).
3. Results
(a) Relative Acp transcript abundance
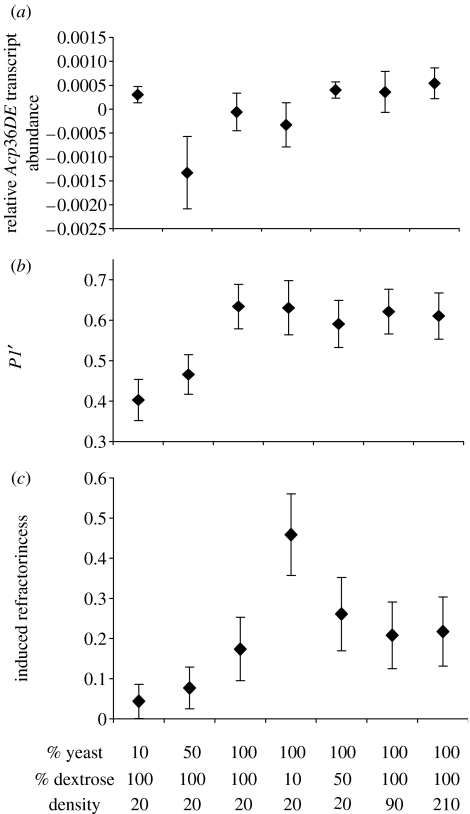
RPL32 transcript abundance did not differ between treatments, but was positively correlated with transcript abundances of all nine Acp genes (r2=0.54–0.97). Larval culture density had no effect on the relative transcript abundance of any of the tested Acps, while larval nutrient availability affected the relative transcript abundance of Acp36DE (F4,25=4.658, p=0.006). Separate one-way ANOVAs revealed that larval dextrose availability had no effect on the relative transcript abundance of Acp36DE, but males reared on 50% yeast had reduced relative levels of Acp36DE transcript when compared with controls (F2,15=8.0645, p=0.004; figure 1a).
Figure 1.
Means and standard errors of the effects of larval rearing conditions on (a) relative Acp36DE transcript abundance given as the residual 1/CT value regressed against a control gene, RPL32, (b) P1′ and (c) female refractoriness. Larval rearing conditions are shown on the x-axis. Percentage of yeast and dextrose is given relative to the control (100%). Density is given as the number of larvae per vial.
(b) Sperm competition phenotypes
Larval culture density did not affect any of the measured sperm competition phenotypes (N=56 females; p>0.30 for all phenotypes). In contrast, nutrient availability during larval life affected P1′ (F4,94=3.19; p=0.017; marginally significant after sequential Bonferroni correction) and female refractoriness (N=119 females in five treatments; p=0.015 from permutation test). Larval nutrition did not affect female fecundity (F4,90=1.00; p=0.41) nor did larval yeast availability affect female productivity (F2,75=1.64; p=0.20). Separate one-way ANOVAs revealed that dextrose levels in larval medium had no effect on P1′ (F2,46=1.30; p=0.282), but yeast levels did (F2,62=3.89; p=0.026; significant after Bonferroni correction). Males reared on 10% yeast sired a lower proportion of offspring when compared with controls (Tukey test, p=0.023; figure 1b). Pairwise comparisons among all treatments revealed that female refractoriness was marginally elevated when mated to males reared on 10% dextrose (0.46±0.10 s.e. versus 0.17±0.08 s.e.; p=0.03; figure 1c) and was marginally reduced when mated to males reared on 10% yeast (0.04±0.04 s.e. versus 0.17±0.08 s.e.; p=0.05; figure 1c) when compared with controls. In addition, significant differences in refractoriness were detected between females mated to males reared on 10% dextrose versus males reared on either 10% yeast (0.46±0.10 s.e. versus 0.04±0.04 s.e.; p=0.0007; figure 1c) or 50% yeast (0.46±0.10 s.e. versus 0.08±0.05 s.e.; p=0.001; figure 1c).
(c) Sperm transfer and storage
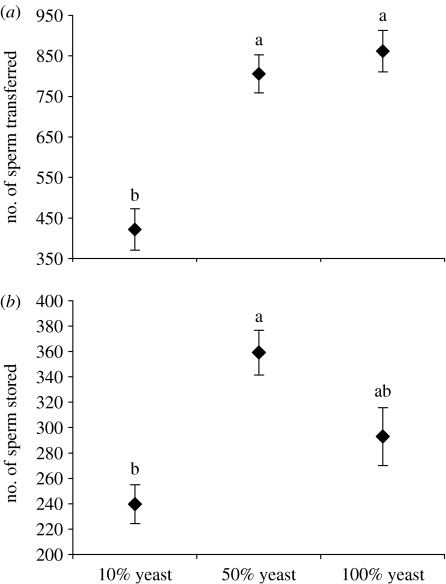
Larval yeast availability significantly affected the amount of sperm males transferred to females (F2,29=22.43, p<0.0001; figure 2a). Males reared on 10% yeast transferred fewer sperm than control males or those reared on 50% yeast (Tukey test, p<0.0001 for both cases; figure 2a). Larval yeast availability also significantly affected the number of sperm stored in the female's seminal receptacles (F2,34=5.5423, p=0.0083; figure 2b). Females retained fewer sperm when mated to males reared on 10% yeast when compared with males reared on 50% yeast, but not control males (Tukey test, p=0.0113; figure 2b).
Figure 2.
Means and standard errors of (a) the number of sperm transferred to females and (b) the number of sperm stored by females from males reared as larvae on medium containing 10, 50 or 100% yeast. Treatments not marked with the same letter are significantly different.
(d) Body size
Larval rearing conditions significantly affected male body size (F6,133=18.61; p<0.001; see table 1 in the electronic supplementary material). Male body size was positively correlated with P1′ (r2=0.62, p=0.034) and this effect appears to be driven by body weights of males reared on 10% yeast (which were significantly smaller than other males). The amount of larval yeast did not significantly affect P1′ after correcting for male body size (F2,64=0.68, p=0.509). Males' body weight was not correlated with sperm transferred or stored, or with the refractoriness of their mates.
4. Discussion
Our manipulations of the male's larval rearing environment led to differences in male body size across treatments with 10% yeast males being the smallest. We found that effects on P1′ were probably a direct result of these differences in body size. Our results, as well as a report that studied other traits (Amitin & Pitnick 2006), suggest that in D. melanogaster, male body size plays a fundamental role in determining the outcome of many post-copulatory processes.
Interestingly, two post-copulatory traits that were reduced when males were reared on 10% yeast (female refractoriness and the number of sperm that females stored) were not correlated with male body size. Of the phenotypes that we measured, these two represent processes over which the female may have the most control (Eberhard 1996) and are physiologically intertwined. Females who have fewer sperm in storage are more likely to remate (reviewed in Bloch Qazi et al. 2003).
We find little evidence that relative Acp transcript abundance depends on the male's larval rearing environment. Larval yeast availability affected the relative transcript levels of only one of the nine tested Acps (Acp36DE). The correlation between the effects of larval environment and relative Acp36DE transcript levels was not linear as expected, but only reduced when males were reared on 50% yeast. Because females require Acp36DE for normal sperm storage (reviewed in Bloch Qazi & Wolfner 2003), we explored whether sperm storage was decreased when females mated to these males. We found that females mated to 50% yeast males store significantly more sperm than 10% yeast males and about the same numbers as control males. Thus, the decrease that we observed in relative Acp36DE transcript abundance when males were reared on 50% yeast does not suggest that these males transfer less Acp36DE to the female. We can currently offer no explanation as to why relative Acp36DE transcript levels were lower when yeast levels were only moderately reduced.
We note that while relative Acp transcript levels were unchanged in most treatments, protein levels may be affected. Thus, it will be of future interest to determine if the larval rearing environment affects the amount of Acp protein transferred to the female.
In summary, our experiments suggest that males' abilities to sequester and allocate resources in response to the larval environment may ultimately affect variation in several traits involved in post-copulatory sexual selection.
Acknowledgments
This work was supported by NIH grant HD38921 to M.F.W., NSF grant DEB-0108965 to A.G.C., NIH training grant T32-07617 for a traineeship to L.A.M. and a NIH post-doctoral fellowship NGA 1 F32 GM70300-01 to A.C.F. We thank K. McKean, L. Rowe and L. Sirot for their helpful comments on the manuscript.
Footnotes
These authors contributed equally to this work.
Supplementary Material
Multiple comparisons (Tukey–Kramer HSD) between groups were performed. Treatments not labelled with the same letter are statistically different from one another
References
- Amitin E.G, Pitnick S. Influence of developmental environment on male- and female-mediated sperm precedence in Drosophila melanogaster. J. Evol. Biol. 2006;20:381–391. doi: 10.1111/j.1420-9101.2006.01184.x. doi:10.1111/j.1420-9101.2006.01184.x [DOI] [PubMed] [Google Scholar]
- Andersson M.B. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Bloch Qazi M.C, Wolfner M.F. An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J. Exp. Biol. 2003;206:3521–3528. doi: 10.1242/jeb.00585. doi:10.1242/jeb.00585 [DOI] [PubMed] [Google Scholar]
- Bloch Qazi M.C, Heifetz Y, Wolfner M.F. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev. Biol. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. doi:10.1016/S0012-1606(02)00125-2 [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Princeton University Press; Princeton, NJ: 1996. Female control: sexual selection by cryptic female choice. [Google Scholar]
- Fiumera A.C, Dumont B.L, Clark A.G. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics. 2005;169:243–257. doi: 10.1534/genetics.104.032870. doi:10.1534/genetics.104.032870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E. Sex-peptides: seminal peptides of the Drosophila male. Cell Mol. Life Sci. 2003;60:1689–1704. doi: 10.1007/s00018-003-3052. doi:10.1007/s00018-003-3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller L.D, Graves J.L, Rose M.R. Interactions between density-dependent and age-specific selection in Drosophila melanogaster. Funct. Ecol. 1993;7:469–479. doi:10.2307/2390034 [Google Scholar]
- Rice W.R. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. doi:10.2307/2409177 [DOI] [PubMed] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B. 1996;263:1415–1421. doi:10.1098/rspb.1996.0207 [Google Scholar]
- Wolfner M.F. The gifts that keep on giving: physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity. 2002;88:85–93. doi: 10.1038/sj.hdy.6800017. doi:10.1038/sj.hdy.6800017 [DOI] [PubMed] [Google Scholar]
- Wolfner M.F, Applebaum S, Heifetz Y. Insect gonadal glands and their gene products. In: Gilbert L, Iatrou K, Gill S, editors. Comprehensive insect physiology, pharmacology and molecular biology. Elsevier; Amsterdam, The Netherlands: 2005. pp. 179–212. [Google Scholar]
- Wong A, Wolfner M.F. Sexual behavior: a seminal peptide stimulates appetites. Curr. Biol. 2006;16:R256–R257. doi: 10.1016/j.cub.2006.03.003. doi:10.1016/j.cub.2006.03.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multiple comparisons (Tukey–Kramer HSD) between groups were performed. Treatments not labelled with the same letter are statistically different from one another