Abstract
Urbanization dramatically changes the composition and diversity of biotic communities. The characteristics distinguishing species that persist in urban environments, however, are poorly understood. Here we test the hypothesis that broadly adapted organisms are better able to tolerate urbanization, using a phylogenetically controlled, global comparison of birds. We compared elevational and latitudinal distributions of 217 urban birds found in 73 of the world's largest cities with distributions of 247 rural congeners to test the hypothesis that urban birds possess broader environmental tolerance. Urban birds had markedly broader environmental tolerance than rural congeners, as estimated by elevational and latitudinal distributions. Our results suggest that broad environmental tolerance may predispose some birds to thrive in urban habitats. The mechanisms mediating such environmental tolerance warrant further investigation, but probably include greater behavioural, physiological and ecological flexibility.
Keywords: environmental tolerance, generalist, specialist, urbanization
1. Introduction
Human-dominated environments are an increasingly prominent feature of the Earth's ecosystems (Vitousek et al. 1997). As habitat becomes altered by urbanization, species assemblages change. Most species do not tolerate urban habitat, but some persist and even thrive in cities. What characteristics differentiate the species that persist from those that cannot? One prominent hypothesis to explain how species respond to habitat disturbance is that organisms with broad environmental tolerance (generalists) are less sensitive to human disturbance than those with a more narrow tolerance (specialists), and thus generalists predominate in disturbed areas (Ricklefs & Cox 1972; Glazier 1986). Empirical data appear to support this idea (Clergeau et al. 1998; Kitahara et al. 2000; Ishitami et al. 2003; Swihart et al. 2003), but the hypothesis has never been adequately tested because previous studies suffer two significant shortcomings: (i) studies were conducted at a local scale, limiting our ability to generalize broadly and/or (ii) studies did not control for phylogeny, making the causes of observed patterns unclear. To address these shortcomings, we present the first global, phylogenetically controlled comparison of urban and rural organisms to test the prediction that broadly adapted species are more likely to be found in cities than more narrowly adapted species.
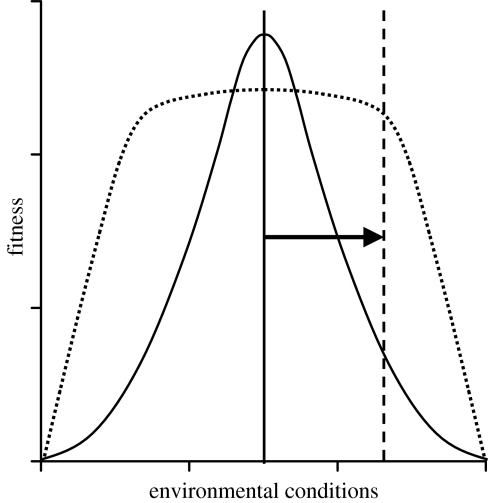
Species vary dramatically in their breadth of environmental tolerance. For example, ecological generalists survive and reproduce across a broad range of climatic conditions, use a diverse array of resources and can be found occupying many distinct habitats. In contrast, ecological specialists may tolerate only a narrow range of climatic conditions, specialize on few resources or occur in a limited range of habitats. Here we define ‘environmental tolerance’ as the ability to survive and reproduce in a given environment. In response to habitat disturbance and alteration, we predicted that species with broad environmental tolerance would fare better than species with a more narrow environmental tolerance because the shift in conditions created by disturbance is more likely to fall within the generalist species' range of tolerance (Glazier 1986; figure 1).
Figure 1.
Illustration of our prediction that urban birds are more likely to be broadly tolerant of environmental conditions than rural congeners. Fitness of a bird with narrow tolerance (solid curve) drops off steeply with any changes in the environment. In contrast, a bird with broad tolerance (dotted curve) has maximum fitness across a wide range of environmental conditions, which drops off at more extreme conditions. If an area where the two species coexist becomes urbanized (arrow), environmental conditions (vertical lines) will shift, and are more likely to fall within the range of tolerance of a bird with broad environmental tolerance than that of a bird with narrow tolerance.
The breadth of an organism's tolerance is difficult to measure directly. A species' geographical distribution can provide a reliable index of environmental tolerance, because variation in environmental conditions increases with latitudinal and elevational distributions, and range size has been found to positively correlate with measures of ecological breadth across a wide array of taxa, including vascular plants (Thompson et al. 1998), insects (Quinn et al. 1997), fishes (Pyron 1999), rodents (Glazier 1980), primates (Harcourt et al. 2002) and birds (Symonds & Johnson 2006; Cofre et al. 2007). Thus, we assume that species with broad geographical ranges are likely to experience a wider range of conditions and have broad environmental tolerance.
We used a dataset of 217 urban and 247 rural birds to test the hypothesis that urban birds have broader environmental tolerance than rural congeners. We used geographical distributions as an index of the breadth of a species' environmental tolerance, and predicted that urban birds would have broader latitudinal and elevational distributions during the breeding season compared with rural congeners.
2. Material and methods
We tested our hypothesis using a dataset of common breeding birds from 73 of the largest cities worldwide (table S1 and figure S1 in the electronic supplementary material). We compiled these data by circulating a questionnaire to ornithologists, biologists and birdwatchers via the Internet (appendix S1 in the electronic supplementary material). Respondents included 54 birdwatchers, 3 biologists and 44 ornithologists. We asked respondents to list up to 10 common native breeding birds found in their city. Reports of bird species occurrence from private citizens have been used previously and found to be accurate (Lepczyk 2005), particularly when analyses do not require complete descriptions of avifauna or assessments of relative abundance of species. Our analyses relied only on an accurate assessment of the presence of a given species breeding in a city, and thus citizen-reported data were expected to be reliable. In several cases, multiple questionnaires were completed for the same cities by independent observers, confirming the presence of some of the same breeding species. For the majority of the species, we also confirmed their use of urban areas for breeding using published habitat descriptions. All urban species also occur outside cities, and we considered the descriptions of their entire breeding distributions in data analyses.
The resulting dataset included 337 native bird species. We reviewed all congeners for each of these species to find candidate rural birds for within-genus comparisons, using a recent global taxonomy (Dickinson 2003). Rural birds had to meet two criteria for inclusion in the analyses: (i) the species could not be described in the literature as breeding in human-disturbed habitat such as towns or cities, and (ii) the bird's breeding distribution must geographically overlap at least one of the cities for which an urban congener was reported as a common native breeder. This second criterion ensures that birds categorized as rural species once probably inhabited the land area now occupied by at least one large city and are absent from that city owing to their inability to persist in urban habitat, rather than other factors that might influence species distributions. We could not pair 120 urban birds within genus, because either they represent monotypic genera (27 species) or none of the congeners met the two criteria as described above (93 species). These species were excluded from subsequent analyses. After excluding these species, our dataset included 217 urban and 247 rural species from 14 orders, 44 families and 100 genera (appendix S2 and table S2 in the electronic supplementary material). Each genus was represented by 2.2 urban and 2.5 rural species on average. Species that were excluded from the analyses because they could not be paired within genus did not differ statistically from included species in elevational or latitudinal range.
We reviewed the published literature and compiled data on elevational and latitudinal distributions during breeding for all species. We restricted data to breeding distributions because we define tolerance as the ability to survive and reproduce in a given environment, and to control for potential confounds associated with differences in migratory tendency. Data on elevational range were not available for nine urban and three rural species. These species were excluded from the analyses. Overall, one genus was excluded from elevational range comparisons owing to missing data.
For statistical analyses, we calculated the mean elevational and latitudinal ranges within each genus for both urban and rural species, and compared these values by calculating a within-genus standardized difference in each parameter as follows: (mean urban value within the genus−mean rural value within the genus)/mean overall value for the genus. Thus, positive values indicated that the elevational or latitudinal range was greater in the urban congener. We then conducted one-tailed, one-sample t-tests for each parameter to determine whether these standardized values were greater than zero.
3. Results
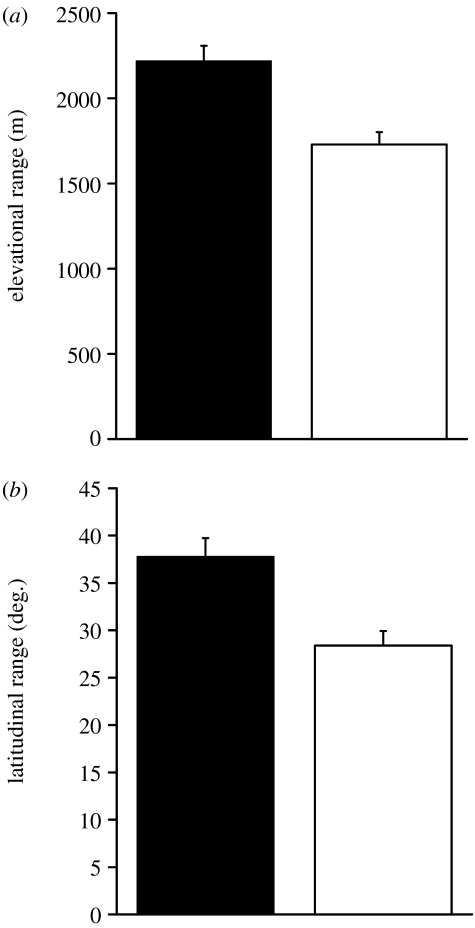
Urban birds had significantly broader elevational and latitudinal distributions than rural congeners (one-tailed, one-sample t-tests of the standardized difference in each parameter within genus; elevation, t=4.60, d.f.=99, p<0.00001; latitude, t=5.42, d.f.=100, p<0.000001; figure 2a,b). Urban birds had elevational ranges that were more than 500 m broader than the ranges of rural birds and latitudinal distributions that were 10° broader than the distributions of rural birds. This pattern remained when analyses were restricted to genera reported in only one city or genera found exclusively in the Americas (appendix S3 in the electronic supplementary material).
Figure 2.
Mean (a) elevational range and (b) latitudinal range of urban (black bars) and rural (open bars) birds. The error bars indicate +1 s.e. Urban birds had broader elevational and latitudinal ranges than rural congeners (one-sample t-tests; elevation, t=4.60, d.f.=99, p<0.00001; latitude, t=5.42, d.f.=100, p<0.000001).
4. Discussion
This study is the first global, phylogenetically controlled comparison of urban and rural birds, which reveals that species that have adapted to a broad array of environmental conditions may be better able to tolerate human-disturbed habitat. Urban birds have markedly broader environmental tolerance than rural congeners, as indicated by their broader elevational and latitudinal distributions during breeding.
Behavioural, physiological and ecological flexibility may contribute to an urban bird's ability to tolerate a broad array of environmental conditions, including disturbed habitat. This flexibility may include traits such as a bird's ability to adjust behaviour in response to novel conditions, to resist detrimental physiological effects of breeding in urban habitat or to use novel resources, such as food types or nest sites. One species of birds was found to adjust singing behaviour in response to urban noise levels (Slabbekoorn & Peet 2003). The physiology of urban birds has only recently received attention, revealing remarkable within-species differences in urban and rural birds (Schoech et al. 2004; Partecke et al. 2006; Bonier et al. 2007). For example, Schoech et al. (2004) found that suburban Florida scrub-jays (Aphelocoma coerulescens) had lower stress hormone levels than rural conspecifics. Behavioural flexibility and the use of novel resources have also been investigated in broadly distributed birds. Sol et al. (2002, 2005) found that relative brain size and frequency of foraging innovations in these birds were positively correlated with a measure of potential for successful invasion into novel habitat. In combination or separately, these and other characteristics of the behaviour, physiology and ecology of urban birds may be keys to their tolerance of a wide array of environments, predisposing them to succeed in human-disturbed habitat. Further study directly measuring environmental tolerance of urban and rural organisms, which was not possible here, could provide a more direct link between urban species and environmental tolerance.
The results of our study suggest that small-ranged specialist species will be impacted more than larger-ranged generalists as urbanization continues. These species are already at risk owing to their relatively small population and range sizes (Purvis et al. 2000). The compounding effects of small population size, small range size and an increased sensitivity to habitat disturbance may put these species at greater risk than previously predicted.
Acknowledgments
We thank Fernando Straube for assistance with translation and circulation of questionnaires in South America, Toby Bradshaw, Martha Groom, Ray Huey, F. Gary Stiles, Fernando Straube and Josh Tewksbury for their comments on the study design, Shallin Busch for helpful comments on an earlier version of the manuscript, Wand Ali and his colleagues in Iraq for their invaluable assistance with the birds of Baghdad, and the 101 people from around the world who responded to our survey.
Supplementary Material
Tables S1 and S2, Figures S1 and S2, and Appendices S1 and S3. Supplementary information regarding cities and taxa sampled, the questionnaire used for data collection, sources used for compiling data, and post-hoc statistical analyses
All urban and rural species included in the study, with reference to the cities for which they were reported
References
- Bonier F, Martin P.R, Sheldon K.S, Jensen J.P, Foltz S.L, Wingfield J.C. Sex-specific consequences of life in the city. Behav. Ecol. 2007;18:121–129. doi:10.1093/beheco/arl050 [Google Scholar]
- Clergeau P, Savard J.-P.L, Mennechez G, Falardeau G. Bird abundance and diversity along an urban–rural gradient: a comparative study between two cities on different continents. Condor. 1998;100:413–425. doi:10.2307/1369707 [Google Scholar]
- Cofre H.L, Böhning-Gaese K, Marquet P.A. Rarity in Chilean forest birds: which ecological and life-history traits matter? Divers. Distrib. 2007;13:203–212. [Google Scholar]
- Dickinson, E. C. (ed.) 2003 The Howard and Moore complete checklist of the birds of the world, 3rd edn. Princeton, NJ: Princeton University Press.
- Glazier D.S. Ecological shifts and the evolution of geographically restricted species of North American Peromyscus (mice) J. Biogeogr. 1980;7:63–83. doi:10.2307/2844547 [Google Scholar]
- Glazier D.S. Temporal variability of abundance and the distribution of species. Oikos. 1986;47:309–314. doi:10.2307/3565442 [Google Scholar]
- Harcourt A.H, Coppeto S.A, Parks S.A. Rarity, specialization and extinction in primates. J. Biogeogr. 2002;29:445–456. doi:10.1046/j.1365-2699.2002.00685.x [Google Scholar]
- Ishitami M, Kotze D.J, Niemelä J. Changes in carabid beetle assemblages across an urban–rural gradient in Japan. Ecography. 2003;26:481–489. doi:10.1034/j.1600-0587.2003.03436.x [Google Scholar]
- Kitahara M, Sei K, Fujii K. Patterns in the structure of grassland butterfly communities along a gradient of human disturbance: further analysis based on the generalist/specialist concept. Popul. Ecol. 2000;42:135–144. doi:10.1007/PL00011992 [Google Scholar]
- Lepczyk C.A. Integrating published data and citizen science to describe bird diversity across a landscape. J. Appl. Ecol. 2005;42:672–677. doi:10.1111/j.1365-2664.2005.01059.x [Google Scholar]
- Partecke J, Schwabl I, Gwinner E. Stress and the city: urbanization and its effects on the stress physiology in European blackbirds. Ecology. 2006;87:1945–1952. doi: 10.1890/0012-9658(2006)87[1945:satcua]2.0.co;2. doi:10.1890/0012-9658(2006)87[1945:SATCUA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Purvis A, Gittleman J.L, Cowlishaw G, Mace G.M. Predicting extinction risk in declining species. Proc. R. Soc. B. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. doi:10.1098/rspb.2000.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyron M. Relationships between geographical range size, body size, local abundance, and habitat breadth in North American suckers and sunfishes. J. Biogeogr. 1999;26:549–558. doi:10.1046/j.1365-2699.1999.00303.x [Google Scholar]
- Quinn R, Gaston K, Blackburn T, Eversham B. Abundance–range size relationships of macrolepidoptera in Britain: the effects of taxonomy and life history variables. Ecol. Entomol. 1997;22:453–461. doi:10.1046/j.1365-2311.1997.00090.x [Google Scholar]
- Ricklefs R.E, Cox G.W. Taxon cycles in the West Indian avifauna. Am. Nat. 1972;106:195. doi:10.1086/282762 [Google Scholar]
- Schoech S.J, Bowman R, Reynolds S.J. Food supplementation and possible mechanisms underlying early breeding in the Florida Scrub-Jay (Aphelocoma coerulescens) Horm. Behav. 2004;46:565–573. doi: 10.1016/j.yhbeh.2004.06.005. doi:10.1016/j.yhbeh.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Peet M. Birds sing at a higher pitch in urban noise. Nature. 2003;424:267. doi: 10.1038/424267a. doi:10.1038/424267a [DOI] [PubMed] [Google Scholar]
- Sol D, Timmermans S, Lefebvre L. Behavioural flexibility and invasion success in birds. Anim. Behav. 2002;63:495–502. doi:10.1006/anbe.2001.1953 [Google Scholar]
- Sol D, Duncan R.P, Blackburn T.M, Cassey P, Lefebvre L. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA. 2005;102:5460–5465. doi: 10.1073/pnas.0408145102. doi:10.1073/pnas.0408145102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swihart R.K, Gehring T.M, Kolozsvary M.B, Nupp T.E. Responses of ‘resistant’ vertebrates to habitat loss and fragmentation: the importance of niche breadth and range boundaries. Divers. Distrib. 2003;9:1–18. doi:10.1046/j.1472-4642.2003.00158.x [Google Scholar]
- Symonds M.R.E, Johnson C.N. Determinants of local abundance in a major radiation of Australian passerines (Aves: Meliphagoidea) J. Biogeogr. 2006;33:794–802. doi:10.1111/j.1365-2699.2005.01432.x [Google Scholar]
- Thompson K, Hodgson J.G, Gaston K.J. Abundance–range size relationships in the herbaceous flora of central England. J. Ecol. 1998;86:439–448. doi:10.1046/j.1365-2745.1998.00264.x [Google Scholar]
- Vitousek P.M, Mooney H.A, Lubchenco J, Melillo J.M. Human domination of Earth's ecosystems. Science. 1997;277:494–499. doi:10.1126/science.277.5325.494 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2, Figures S1 and S2, and Appendices S1 and S3. Supplementary information regarding cities and taxa sampled, the questionnaire used for data collection, sources used for compiling data, and post-hoc statistical analyses
All urban and rural species included in the study, with reference to the cities for which they were reported