Abstract
Juvenile loggerhead sea turtles spend more than a decade in the open ocean before returning to neritic waters to mature and reproduce. It has been assumed that this transition from an oceanic to neritic existence is a discrete ontogenetic niche shift. We tested this hypothesis by tracking the movements of large juveniles collected in a neritic foraging ground in North Carolina, USA. Our work shows that the shift from the oceanic to neritic waters is both complex and reversible; some individuals move back into coastal waters and then return to the open ocean for reasons that are still unclear, sometimes for multiple years. These findings have important consequences for efforts to protect these threatened marine reptiles from mortality in both coastal and open-ocean fisheries.
Keywords: loggerhead turtle, ontogenetic, migration, neritic, oceanic
1. Introduction
Throughout their development, loggerhead sea turtles (Caretta caretta) undergo abrupt developmental changes in habitat, behaviour and resource use (Bjorndal et al. 2000; Snover 2002; Bolten 2003). In the western North Atlantic, hatchlings leave their natal beaches, swim offshore and become entrained into the Gulf Stream, eventually inhabiting the oceanic waters of the Atlantic. Their whereabouts during this oceanic phase of life was termed the ‘lost year’ by Archie Carr (Carr 1986). We now know that this lost year actually lasts for more than a decade, during which the turtles remain in oceanic waters and consume epipelagic prey (Carr 1987; Bjorndal et al. 2000; Snover 2002).
After this oceanic phase, juvenile loggerheads return to neritic waters to forage on benthic prey, in what has been assumed to be a discrete ontogenetic shift. In the North Atlantic, this shift occurs when individuals are 42–59 cm straight carapace length (SCL; Bjorndal et al. 2000; Snover 2002). Once these large juveniles have entered a benthic life stage, they inhabit coastal waters and make seasonal latitudinal migrations between neritic feeding (and later breeding) grounds (Carr 1987; Musick & Limpus 1997; Hopkins-Murphy et al. 2003). This shift is believed to allow maximization of growth potential while minimizing predation risk in ecologically distinct stages (Werner & Gilliam 1984).
Discrete ontogenetic niche shifts are common in vertebrates (Mittelbach 1981), but in some species such shifts are not hard-wired (Persson & Greenberg 1990). The timing of these shifts may have profound consequences for survivorship (Olson 1996) and, in a world that is increasingly modified by human activities, may mediate risk to sources of anthropogenic mortality.
We tested the hypothesis that loggerheads exhibit a permanent ontogenetic shift and once juveniles have returned to neritic waters they remain in this habitat. Loggerhead sea turtle populations are threatened by mortality in both coastal and oceanic fisheries, so it is critical to understand variation in their life histories (Crouse et al. 1987; Crowder et al. 1994) so that we can assess the demographic impacts of these fisheries.
2. Material and methods
We used satellite telemetry to track the movements of 30 large juveniles captured in the estuaries of North Carolina, USA, in the summers of 2002 and 2003. We attached satellite transmitters (Wildlife Computers SPOT2, Redmond, Washington, USA) to loggerhead turtles collected incidentally in a commercial pound net fishery in North Carolina. Turtle size was measured to the nearest 0.1 cm and reported as SCL. Transmitters were affixed to the turtle's carapace using a hybrid technique of a cool setting two-part epoxy (Power Fast; Mitchell 1998) and fibreglass cloth and resin (Balazs et al. 1996). Animals were released near their capture location. The satellite tags were programmed to transmit daily over an 8 h period beginning from just before dawn to near midday. Position estimates and associated location accuracy were provided by Service Argos, Inc. We employed a three-stage filtering algorithm (McConnell et al. 1992; Austin et al. 2003) to reject implausible locations. Sea turtle movements were reconstructed by plotting the best location estimate per day of the filtered location data. To test for significant differences in the size of turtles with different migratory destinations, we conducted a one-way analysis of variance. Migratory destination was classified as ‘oceanic’ if a turtle moved off the continental shelf, as defined by the 200 m isobath, or ‘neritic’ if it remained on the shelf. Seven turtles tracked for less than one month and, showing no clear destination after leaving the estuaries, were excluded from the analysis.
3. Results
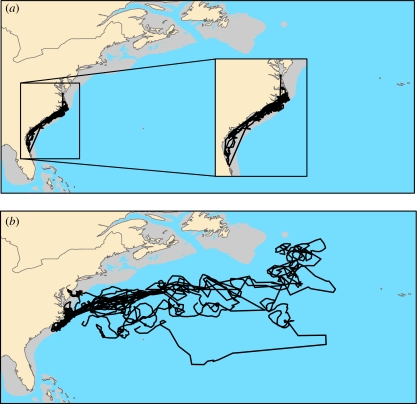
Turtles were tracked for up to 660 days (mean=274; s.d.=108) and exhibited two discrete behavioural patterns. Thirteen turtles remained in neritic waters as expected; some travelled as far south as Florida, although most remained off North Carolina (figure 1a). The following year, 10 of these loggerheads returned to North Carolina estuaries. The three remaining individuals stopped transmitting prior to or around the time remigration typically occurs; one in January off Florida and two in April off North Carolina.
Figure 1.
Two discrete life-history patterns observed in juvenile loggerhead sea turtles tracked via satellite from a summer estuarine foraging area: (a) neritic and (b) oceanic. Neritic animals (n=13) remained over the continental shelf (in grey). Oceanic turtles (n=10) ventured past the shelf break into the open ocean.
After leaving the estuaries, however, a group of 10 turtles returned to the open ocean (figure 1b), where they remained for up to 381 days. Loggerheads cannot feed on benthic organisms in this habitat because water depths exceed their dive capabilities (Lutcavage & Lutz 1997). One turtle, after spending 210 days in the oceanic realm, returned to coastal waters for 102 days before migrating back to the open ocean for 30 days, after which its transmissions ceased.
The mean SCL of neritic turtles was 62.6 cm (s.d.=4.9, range=57.5–75.8), whereas that of oceanic turtles was 64.0 cm SCL (s.d.=7.3, range=52.9–74.2). Their sizes were not significantly different (F1,21=0.32, p=0.58).
4. Discussion
Our results show that loggerheads do not undergo a discrete ontogenetic niche shift and add to speculation (Bolten 2003) and a growing body of evidence (Witzell 2002; Hatase et al. 2006; Hawkes et al. 2006) that the life history of sea turtles is considerably more complex than previously understood. Tracking individuals in the wild has provided considerable insight into movement behaviours, but individual variation may not always be recognized as significant. For example, in two decades of satellite telemetry studies, a few loggerheads were tracked into the Gulf Stream for short periods of time (Byles 1988; Keinath 1993; Morreale & Standora 1998), but these results were, for the most part, discounted as anomalous. Morreale & Standora (2005) later suggested that some juvenile loggerheads migrate seasonally between coastal habitats and warmer offshore waters. Our study demonstrates clearly that the oceanic environment remains an important habitat for loggerheads even after they venture into neritic foraging grounds, sometimes for several years. Indeed, large juveniles such as those in our study are routinely observed as by-catch in pelagic longline fisheries in oceanic waters (Watson et al. 2005).
The factors responsible for this observed variation in life-history strategies are not yet clear. Size did not explain the difference in migratory destination in this study and we are not yet able to assess the influence of other potential explanatory factors such as age, sex, condition or nesting beach origin. We can infer, however, from other research that these differences are unlikely to explain the variation we observed. Juvenile loggerheads captured in North Carolina are a mixed-stock aggregation (Bowen et al. 2005), although most are from western Atlantic rookeries (Bass et al. 2004), and are female biased (2 : 1; Braun-McNeill et al. in press).
Using skeletochronology and stable isotopes, Snover (2002) suggested that loggerheads settled into the neritic environment at 49 cm SCL. She found increased growth rates and a trophic shift following settlement that was consistent with a transition from pelagic to benthic habitat and prey, but reported that growth rates in the neritic habitat were slower than predicted (Snover 2002; Snover et al. 2007). The behavioural complexities we describe in terms of life-history strategy may not be evident in such studies because of the observed variation in growth or diet. In particular, we conclude that ontogenetic shifts exhibited by loggerhead turtles appear to be facultative and reversible, rather than fixed.
The intrapopulation variation we observed may have significant consequences for both the description of sea turtle life histories and for the conservation of these protected species. Sea turtle by-catch in fisheries that occur within the exclusive economic zones of nations can be mitigated (e.g. the use of Turtle Excluder Devices in US trawl fisheries), but when loggerheads spend protracted periods in the ocean, they are at risk from largely unregulated international fisheries (e.g. pelagic longlines for swordfish and tuna). Our study suggests that as many as one-third of the demographically critical large juvenile loggerheads (Crowder et al. 1994) may remain vulnerable to fisheries by-catch in the open ocean much longer than previously understood.
Acknowledgements
Research on sea turtles was authorized by NMFS Scientific Research Permit no. 1260 and the Duke University Institutional Animal Care and Use Committee (A146-02-05).
We thank Joanne McNeill, Sheryan Epperly and the National Marine Fisheries Service Beaufort, NC laboratory for logistical support, and Danielle Waples, many volunteers and the commercial fishermen for help in the field. We are grateful to Matthew Godfrey, Larry Crowder and two referees for constructive comments on the manuscript. This work was supported by NC Sea Grant Fisheries Resource Grant 02-FEG-05 and the Oak Foundation.
References
- Austin D, McMillan J.I, Bowen W.D. A three-stage algorithm for filtering erroneous Argos satellite locations. Mar. Mamm. Sci. 2003;19:371–383. doi:10.1111/j.1748-7692.2003.tb01115.x [Google Scholar]
- Balazs, G. H., Miya, R. K. & Beavers, S. C. 1996 Procedures to attach a satellite transmitter to the carapace of an adult green turtle, Chelonia mydas NOAA technical memorandum NMFS-SEFSC-387, pp. 21–26. Miami, FL: National Marine Fisheries Service, Southeast Fisheries Science Center.
- Bass A.L, Epperly S.P, Braun-McNeill J. Multi-year analysis of stock composition of a loggerhead turtle (Caretta caretta) foraging habitat using maximum likelihood and Bayesian methods. Conserv. Genet. 2004;5:783–796. doi:10.1007/s10592-004-1979-1 [Google Scholar]
- Bjorndal K.A, Bolten A.B, Martins H.R. Somatic growth model of juvenile loggerhead sea turtles Caretta caretta: duration of pelagic stage. Mar. Ecol. Prog. Ser. 2000;202:265–272. [Google Scholar]
- Bolten A.B. Active swimmers—passive drifters: the oceanic juvenile stage of loggerheads in the Atlantic system. In: Bolten A.B, Witherington B.E, editors. Loggerhead sea turtles. Smithsonian Books; Washington, DC: 2003. pp. 63–78. [Google Scholar]
- Bowen B.W, Bass A.L, Soares L, Tooneen R.J. Conservation implications of complex population structure: lessons from the loggerhead turtle (Caretta caretta) Mol. Ecol. 2005;14:2389–2402. doi: 10.1111/j.1365-294X.2005.02598.x. doi:10.1111/j.1365-294X.2005.02598.x [DOI] [PubMed] [Google Scholar]
- Braun-McNeill, J., Epperly, S. P., Owens, D. W., Avens, L., Williams, E. & Harms, C. A. In press. Seasonal reliability of testosterone radioimmunoassay (RIA) for predicting sex ratios of juvenile loggerhead (Caretta caretta) turtles. Herpetol. Rev
- Byles, R. A. 1988 The behavior and ecology of sea turtles in Virginia. PhD dissertation, College of William and Mary, Gloucester Point, VA.
- Carr A.F. Rips, FADS, and little loggerheads. BioScience. 1986;36:92–100. doi:10.2307/1310109 [Google Scholar]
- Carr A.F. New perspectives on the pelagic stage of sea turtle development. Conserv. Biol. 1987;1:103–121. doi:10.1111/j.1523-1739.1987.tb00020.x [Google Scholar]
- Crouse D.T, Crowder L.B, Caswell H. A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology. 1987;68:1412–1423. doi:10.2307/1939225 [Google Scholar]
- Crowder L.B, Crouse D.T, Heppell S.S, Martin T.M. Predicting the impact of turtle excluder devices on the loggerhead sea turtle populations. Ecol. Appl. 1994;4:437–445. doi:10.2307/1941948 [Google Scholar]
- Hatase H, Sato K, Yamaguchi M, Takahashi K, Tsukamoto K. Individual variation in feeding habitat use by adult female green sea turtles (Chelonia mydas): are they obligately neritic herbivores? Oecologia. 2006;149:52–64. doi: 10.1007/s00442-006-0431-2. doi:10.1007/s00442-006-0431-2 [DOI] [PubMed] [Google Scholar]
- Hawkes L.A, Broderick A.C, Coyne M.S, Godfrey M.H, Lopez-Jurado L.F, Lopez-Suarez P, Merino S.E, Varo-Cruz N, Godley B.J. Phenotypically linked dichotomy in sea turtle foraging requires multiple conservation approaches. Curr. Biol. 2006;16:990–995. doi: 10.1016/j.cub.2006.03.063. doi:10.1016/j.cub.2006.03.063 [DOI] [PubMed] [Google Scholar]
- Hopkins-Murphy S.R, Owens D.W, Murphy T.M. Ecology of immature loggerheads on foraging grounds and adults in internesting habitat in the eastern United States. In: Bolten A.B, Witherington B.E, editors. Loggerhead sea turtles. Smithsonian Institution Press; Washington, DC: 2003. pp. 79–92. [Google Scholar]
- Keinath, J. A. 1993 Movements and behavior of wild and head-started sea turtles. PhD dissertation, College of William and Mary, Gloucester Point, VA.
- Lutcavage M.E, Lutz P.L. Diving physiology. In: Lutz P.L, Musick J.A, editors. The biology of sea turtles. CRC Press; Boca Raton, FL: 1997. pp. 277–296. [Google Scholar]
- McConnell B.J, Chambers J.C, Fedak M.A. Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarct. Sci. 1992;4:393–398. [Google Scholar]
- Mitchell S.V. NOAA technical memorandum NMFS-SEFSC-436. National Marine Fisheries Service, Southeast Fisheries Science Center; Miami, FL: 1998. Use of epoxy in telemeter attachment. [Google Scholar]
- Mittelbach G.G. Foraging efficiency and body size: a study of optimal diet and habitat use by bluegills. Ecology. 1981;62:1370–1386. doi:10.2307/1937300 [Google Scholar]
- Morreale S.J, Standora E.A. NOAA technical memorandum NMFS-SEFSC-413. National Marine Fisheries Service, Southeast Fisheries Science Center; Miami, FL: 1998. Early life stage ecology of sea turtles in northeastern US waters. [Google Scholar]
- Morreale S.J, Standora E.A. Western North Atlantic waters: crucial developmental habitat for Kemp's ridley and loggerhead sea turtles. Chelonian Conserv. Biol. 2005;4:872–882. [Google Scholar]
- Musick J.A, Limpus C.A. Habitat utilization and migration in juvenile sea turtles. In: Lutz P.L, Musick J.A, editors. The Biology of Sea Turtles. CRC Press; Boca Raton, FL: 1997. pp. 137–163. [Google Scholar]
- Olson M.H. Ontogenetic niche shifts in largemouth bass: variability and consequences for first-year growth. Ecology. 1996;77:179–190. doi:10.2307/2265667 [Google Scholar]
- Persson L, Greenberg L.A. Optimal foraging and habitat shift in perch Perca fluviatilis in a resource gradient. Ecology. 1990;71:1699–1713. doi:10.2307/1937579 [Google Scholar]
- Snover, M. L. 2002 Growth and ontogeny of sea turtles using skeletochronology: methods, validation, and applications to conservation. Dissertation, Duke University, Durham, NC.
- Snover M.L, Avens L, Hohn A.A. Back-calculating length from skeletal growth marks in loggerhead sea turtles (Caretta caretta) Endang. Species Res. 2007;3:95–104. [Google Scholar]
- Watson J.W, Epperly S.P, Shah A.K, Foster D.G. Fishing methods to reduce sea turtle mortality associated with pelagic longlines. Can. J. Fish. Aquat. Sci. 2005;62:965–981. doi:10.1139/f05-004 [Google Scholar]
- Werner E.E, Gilliam J.F. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 1984;15:393–425. doi:10.1146/annurev.es.15.110184.002141 [Google Scholar]
- Witzell W.N. Immature Atlantic loggerhead turtles (Caretta caretta): suggested changes to the life history model. Herpetol. Rev. 2002;33:266–269. [Google Scholar]