Abstract
The proximate mechanisms underlying the evolution and maintenance of within-sex variation in mating behaviour are still poorly understood. Species characterized by alternative reproductive tactics provide ideal opportunities to investigate such mechanisms. Bluegill (Lepomis macrochirus) are noteworthy in this regard because they exhibit two distinct cuckolder (parasitic) morphs (called sneaker and satellite) in addition to the parental males that court females. Here we confirm previous findings that spawning cuckolder and parental males have significantly different levels of testosterone and 11-ketotestosterone. We also report, for the first time, that oestradiol and cortisol levels are higher in cuckolders than in parental males. The two cuckolder morphs did not differ in average levels of any of the four hormones. However, among satellite males which mimic females in appearance and behaviour, there was a strong negative relationship between oestradiol levels and body length, a surrogate for age. This finding suggests that for satellite males, oestradiol dependency of mating behaviour decreases with increasing mating experience. Although such decreased hormone dependence of mating behaviour has been reported in other taxa, our data represent the first suggestion of the relationship in fishes.
Keywords: alternative reproductive tactics, Lepomis, cortisol, estradiol, androgens, female mimicry
1. Introduction
Distinct male alternative reproductive tactics (ARTs) have been described in a variety of taxa including fishes (Taborsky 1994). Species with ARTs typically have two specialized morphs that use either a territorial tactic or a sneaking tactic during breeding. Initial research into ARTs focused on game theory and sperm competition (reviewed in Parker 1990; Gross 1996; Taborsky 1998; see also Fu et al. 2001). More recently, the physiological mechanisms contributing to the development and expression of the morphs have received close attention, especially the contribution of chemical messengers that could mediate behavioural expression (Moore 1991; Oliveira 2006; Bass & Forlano in press).
Research into endocrine mechanisms underlying male ARTs initially focused on androgen levels and several studies documented morph differences (reviewed by Moore 1991; Brantley et al. 1993; Knapp 2003; Oliveira 2006). More recently, contributions of oestrogens have been documented. For example, production of oestrogens from androgens via aromatase in behaviourally relevant brain regions differs between ARTs in plainfin midshipman fish (Porichthys notatus; Schlinger et al. 1999; Forlano & Bass 2005). However, circulating levels of oestradiol have been investigated only in a few species (e.g. Oliveira 2006). Also, surprisingly, little attention has been given to the role of glucocorticoids despite potentially large differences in the energetic costs of the various tactics (Knapp 2003), and the possibility that glucocorticoids may thus shed light on whether male morphs in particular species represent evolutionarily stable strategies or are instead ‘making the best of a bad situation’ (Gross 1996; Moore et al. 1998).
In the present study, we determined circulating levels of four steroid hormones in spawning male bluegill that adopt one of three ARTs. Males from our study population are characterized by a discrete life history called ‘parental’ and ‘cuckolder’ (Gross & Charnov 1980). Parental males mature at about the age of 7 years and compete with one another for nesting sites in colonies, court and spawn with females, and provide sole parental care for the offspring (Gross 1982). Cuckolder males mature precociously at approximately 2 years of age and use a parasitic tactic to steal fertilizations from parental males. When small (approx. 2–3 years of age), cuckolder males (‘sneakers’) use a sneaking tactic to streak spawn, and when large (approx. 4–5 years of age), cuckolder males (‘satellites’) use female mimicry to gain access to nests (Dominey 1980; Gross 1982). Because a previous study (Kindler et al. 1989) found no difference in androgen levels between the two cuckolder morphs, we hypothesized that oestradiol or cortisol may contribute to the profound differences in spawning behaviour between sneaker and satellite males for the reasons outlined above.
2. Material and methods
Detailed methods are provided in the electronic supplementary material. Briefly, fish were collected from a spawning colony on 14 June 2004. Males were assigned to reproductive tactic based on direct observations of spawning behaviour, then were collected by hand net and immediately brought to a boat where blood sampling occurred. Only data from samples collected within 180 s were used to avoid confounding baseline cortisol levels with a stress response. Fish were then measured for body size.
Plasma hormone levels were measured using radioimmunoassay (see Magee et al. 2006 for details). Each sample was assayed in duplicate. For oestradiol, a few samples were considered technically ‘non-detectable’ because they fell outside the steep portion of the sigmoid standard curve. However, the standard curve equation still allowed estimation of the low oestradiol levels for these samples. Data were analysed using systat v. 11 (San Jose, CA) as outlined in the electronic supplementary material.
3. Results and discussion
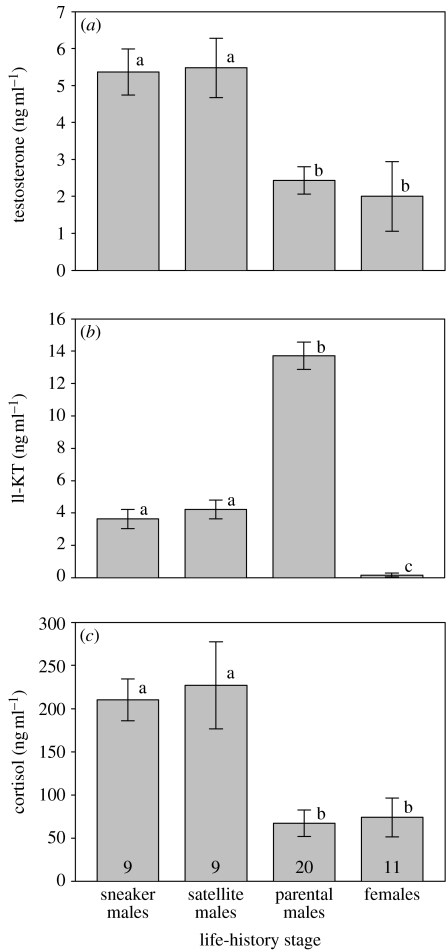
As documented previously by Kindler et al. (1989), courting parental males had significantly higher levels of 11-ketotestosterone (11-KT) than cuckolder males and females (ANOVA: F3,45=73.28, p<0.001; figure 1). This pattern of 11-KT levels is consistent with other fishes with ARTs (Brantley et al. 1993; Oliveira 2006). In contrast, cuckolder males had significantly higher testosterone levels than parental males and females (F3,45=7.63, p<0.001; figure 1). This result differs from Kindler et al. (1989), who found no significant difference among the male morphs. The reason for the discrepancy between studies is unclear, but may be related to variation in social environment (see Oliveira 2004).
Figure 1.
Mean (±s.e.) plasma androgen ((a) testosterone, (b) 11-KT) and (c) cortisol levels for the three male ARTs and females in bluegill on the day of spawning. Groups with different letters differ significantly from each other (post hoc Tukey's tests). Numbers in bars represent sample sizes.
Cuckolder males also had significantly higher cortisol levels than parental males and females (F3,45=10.32, p<0.001; figure 1). This result suggests that cuckolder spawning behaviours are energetically more expensive or otherwise more stressful than courtship and spawning by parental males. However, reproduction by parental males is likely to be more energetically expensive overall when one considers the energetic costs of nest building and parental care. For example, we previously found that parental males' cortisol levels are low on the day of spawning relative to that during nest construction and the week-long care of the young (Magee et al. 2006). Parental males can also lose 10% of their body mass over the course of parental care (Coleman & Fischer 1991; Magee et al. 2006). Our glucocorticoid data from free-living animals are the first from fishes with ARTs and add only to a few other studies of vertebrates with ARTs (e.g. Mendonça et al. 1985; Leary et al. 2004). Hormone manipulation studies on tree lizards (Urosaurus ornatus) and plainfin midshipman have shown that the parasitic morphs are more sensitive to corticosterone than are the displaying morphs (Knapp & Moore 1997; Remage-Healey & Bass 2007). Thus, although it is premature to draw firm conclusions, it appears that glucocorticoids may play a larger role in parasitic morphs than in displaying or territorial males and thereby could factor into the costs and benefits of the various alternative tactics.
We also found that bluegill males' oestradiol levels were low relative to those of females, but cuckolder males had higher levels than parental males (binomial test: p=0.02; table 1). Oestradiol levels are currently known from only five other fish species with two ARTs (Oliveira 2006). In two species, the pattern matches what we report here; in three species, oestradiol levels did not differ significantly between the morphs. Oestradiol's involvement in the expression of ARTs is also supported by neurological responses to oestradiol and morph differences in brain aromatase activity in plainfin midshipman (Schlinger et al. 1999; Forlano & Bass 2005; Remage-Healey & Bass 2007; but see Gonçalves et al. 2007).
Table 1.
Plasma oestradiol levels for the male ARTs and females in bluegill on the day of spawning.
| no. samples | ||||
|---|---|---|---|---|
| n | detectable | non-detectable | median level (ng ml−1) | |
| sneaker males | 9 | 6 | 3 | 0.18 |
| satellite males | 9 | 8 | 1 | 0.25 |
| parental males | 20 | 10 | 10a | 0.05 |
| females | 11 | 11 | 0 | 1.98 |
Significantly different from proportion of cuckolder (sneaker+satellite) samples with detectable hormone levels (one-tailed binomial test: p=0.02).
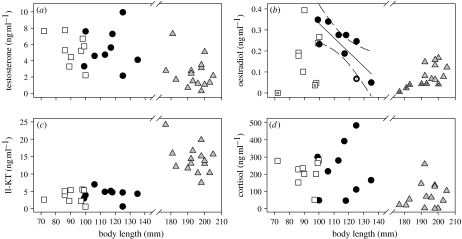
We also explored the hormone dependency of behaviour. In this population of bluegill, as in many fishes, body length is highly correlated with age (Gross & Charnov 1980). We now report a significant negative relationship between body length and oestradiol for satellite males (figure 2). There was no other significant relationship with body length for any other hormones or male morphs (table 2). This finding suggests that there is a diminished dependency of female mimicry behaviour on oestradiol with increased mating experience. We cannot, however, rule out that the negative relationship between oestradiol levels and body length (age) instead reflects an increase in oestradiol receptor concentrations and, hence, an increased sensitivity to oestradiol levels in older satellite males. Regardless, our data support that the endocrine mediation of mating behaviour in satellite males changes with age and experience. Because decreases in hormonal dependency of mating behaviour with experience have been reported for males from several mammalian species (Hull et al. 2002), our finding opens exciting avenues for future research into the interplay between endocrinology and mating experience in ARTs.
Figure 2.
Relationships between body length and plasma hormone levels (ng ml−1) for the male ARTs in bluegill: (a) testosterone, (b) oestradiol, (c) 11-KT and (d) cortisol. Sneakers are represented by squares, satellites are represented by circles and parentals are represented by triangles. The one line (and 95% CI) is from the only significant linear regression (table 2). Dotted data points for oestradiol represent estimates of hormone levels that were below the level of detectability of our assay (see table 2).
Table 2.
Results for simple linear regression analyses of body length versus plasma hormone level for the male ARTs in bluegill. (n.d., not determined because half of the parental males had oestradiol levels below the level of detectability in the radioimmunoassay. We did not correct for multiple comparisons because several pairs of hormone levels are connected via steroidogenic enzymes. Thus, each correlation with body length does not represent an independent test as assumed by common corrections (see electronic supplementary material for additional information). Italics indicate relationship where p<0.05.)
| sneaker males (n=9) | satellite males (n=9) | parental males (n=15) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| hormone | R2 | F1,7 | p | R2 | F1,7 | p | R2 | F1,13 | p |
| testosterone | 0.245 | 2.28 | 0.175 | 0.000 | 0.00 | 0.984 | 0.092 | 1.31 | 0.272 |
| 11-ketotestosterone | 0.000 | 0.00 | 0.955 | 0.027 | 0.19 | 0.674 | 0.180 | 2.86 | 0.114 |
| oestradiol | 0.089 | 0.68 | 0.435 | 0.599 | 10.46 | 0.014 | n.d. | ||
| cortisol | 0.042 | 0.31 | 0.596 | 0.010 | 0.07 | 0.800 | 0.006 | 0.08 | 0.779 |
Acknowledgments
The work conformed to guidelines of the Canadian Council on Animal Care and was approved by the University of Oklahoma Animal Care and Use Committee.
We thank Jeff Stoltz, Tim Hain, Sunny Scobell, Elizabeth Adkins-Regan, Frank Phelan, Floyd Connor and Rich Broughton. This study was supported by funding from the Natural Sciences and Engineering Research Council of Canada and a Premier's Research Excellence Award to B.D.N., and the National Science Foundation (IBN 0349449) and a University of Oklahoma Presidential International Travel Fellowship to R.K.
Supplementary Material
Word file containing details of the methods used for determination of hormone levels and statistical analysis of results
References
- Bass, A. H. & Forlano, P. M. In press. Neuroendocrine mechanisms of alternative reproductive tactics: the chemical language of social plasticity. In Alternative reproductive tactics—an integrative approach (eds R. F. Oliveira, M. Taborsky & J. Brockmann). Cambridge, UK: Cambridge University Press.
- Brantley R.K, Wingfield J.C, Bass A.H. Sex steroid levels in Porichthys notatus, a fish with alternative reproductive tactics, and a review of the hormonal bases for male dimorphism among teleost fish. Horm. Behav. 1993;27:332–347. doi: 10.1006/hbeh.1993.1025. doi:10.1006/hbeh.1993.1025 [DOI] [PubMed] [Google Scholar]
- Coleman R.M, Fischer R.U. Brood size, male fanning effort and the energetics of a non-shareable parental investment in bluegill sunfish, Lepomis macrochirus. Ethology. 1991;87:177–188. [Google Scholar]
- Dominey W.J. Female mimicry in male bluegill sunfish—a genetic polymorphism? Nature. 1980;284:546–548. doi:10.1038/284546a0 [Google Scholar]
- Forlano P.M, Bass A.H. Seasonal plasticity of brain aromatase mRNA expression in glia: divergence across sex and vocal phenotypes. J. Neurobiol. 2005;65:37–49. doi: 10.1002/neu.20179. doi:10.1002/neu.20179 [DOI] [PubMed] [Google Scholar]
- Fu P, Neff B.D, Gross M.R. Tactic-specific success in sperm competition. Proc. R. Soc. B. 2001;268:1105–1112. doi: 10.1098/rspb.2001.1625. doi:10.1098/rspb.2001.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves D, Alpedrinha J, Teles M, Oliveira R.F. Endocrine control of sexual behavior in sneaker males of the peacock blenny Salaria pavo: effects of castration, aromatase inhibition, testosterone and estradiol. Horm. Behav. 2007;51:534–541. doi: 10.1016/j.yhbeh.2007.02.003. doi:10.1016/j.yhbeh.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Gross M.R. Sneakers, satellites and parentals—polymorphic mating strategies in North American sunfishes. Z. Tierpsychol. 1982;60:1–26. [Google Scholar]
- Gross M.R. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 1996;11:92–98. doi: 10.1016/0169-5347(96)81050-0. doi:10.1016/0169-5347(96)81050-0 [DOI] [PubMed] [Google Scholar]
- Gross M.R, Charnov E.L. Alternative male life histories in bluegill sunfish. Proc. Natl Acad. Sci. USA. 1980;77:6937–6940. doi: 10.1073/pnas.77.11.6937. doi:10.1073/pnas.77.11.6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull E.M, Meisel R.L, Sachs B.D. Male sexual behavior. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. Academic Press; San Diego, CA: 2002. pp. 3–137. [Google Scholar]
- Kindler P.M, Philipp D.P, Gross M.R, Bahr J.M. Serum 11-ketotestosterone and testosterone concentrations associated with reproduction in male bluegill (Lepomis macrochirus: Centrarchidae) Gen. Comp. Endocrinol. 1989;75:446–453. doi: 10.1016/0016-6480(89)90180-9. doi:10.1016/0016-6480(89)90180-9 [DOI] [PubMed] [Google Scholar]
- Knapp R. Endocrine mediation of vertebrate male alternative reproductive tactics: the next generation of studies. Integr. Comp. Biol. 2003;43:658–668. doi: 10.1093/icb/43.5.658. doi:10.1093/icb/43.5.658 [DOI] [PubMed] [Google Scholar]
- Knapp R, Moore M.C. Male morphs in tree lizards have different testosterone responses to elevated levels of corticosterone. Gen. Comp. Endocrinol. 1997;107:273–279. doi: 10.1006/gcen.1997.6923. doi:10.1006/gcen.1997.6923 [DOI] [PubMed] [Google Scholar]
- Leary C.J, Jessop T.S, Garcia A.M, Knapp R. Steroid hormone profiles and relative body condition of calling and satellite toads: implications for proximate regulation of behavior in anurans. Behav. Ecol. 2004;15:313–320. doi:10.1093/beheco/arh015 [Google Scholar]
- Magee S.M, Neff B.D, Knapp R. Plasma levels of androgens and cortisol in relation to breeding behavior in parental male bluegill sunfish, Lepomis macrochirus. Horm. Behav. 2006;49:598–609. doi: 10.1016/j.yhbeh.2005.12.003. doi:10.1016/j.yhbeh.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Mendonça M.T, Licht P, Ryan M.J, Barnes R. Changes in hormone levels in relation to breeding behavior in male bullfrogs (Rana catesbiana) at the individual and population levels. Gen. Comp. Endocrinol. 1985;58:270–279. doi: 10.1016/0016-6480(85)90343-0. doi:10.1016/0016-6480(85)90343-0 [DOI] [PubMed] [Google Scholar]
- Moore M.C. Application of organization–activation theory to alternative male reproductive strategies: a review. Horm. Behav. 1991;25:154–179. doi: 10.1016/0018-506x(91)90048-m. doi:10.1016/0018-506X(91)90048-M [DOI] [PubMed] [Google Scholar]
- Moore M.C, Hews D.K, Knapp R. Hormonal control and evolution of alternative male phenotypes: generalizations of models for sexual differentiation. Am. Zool. 1998;38:133–151. [Google Scholar]
- Oliveira R.F. Social modulation of androgens in vertebrates: mechanisms and functions. Adv. Study Behav. 2004;34:165–239. doi:10.1016/S0065-3454(04)34005-2 [Google Scholar]
- Oliveira R.F. Neuroendocrine mechanisms of alternative reproductive tactics in fish. Behav. Physiol. Fish. 2006;24:297–357. doi:10.1016/S1546-5098(05)24008-6 [Google Scholar]
- Parker G.A. Sperm competition games: raffles and roles. Proc. R. Soc. B. 1990;242:120–126. doi:10.1098/rspb.1990.0114 [Google Scholar]
- Remage-Healey L, Bass A.H. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J. Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. doi:10.1523/JNEUROSCI.4282-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger B.A, Greco C, Bass A.H. Aromatase activity in the hindbrain vocal control region of a teleost fish: divergence among males with alternative reproductive tactics. Proc. R. Soc. B. 1999;266:131–136. doi:10.1098/rspb.1999.0612 [Google Scholar]
- Taborsky M. Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv. Study Behav. 1994;23:1–100. [Google Scholar]
- Taborsky M. Sperm competition in fish: ‘bourgeois’ males and parasitic spawning. Trends Ecol. Evol. 1998;13:222–227. doi: 10.1016/s0169-5347(97)01318-9. doi:10.1016/S0169-5347(97)01318-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Word file containing details of the methods used for determination of hormone levels and statistical analysis of results