Abstract
Protected areas form crucial baselines to judge ecological change, yet areas of Africa, Asia and North America that retain large carnivores are under intense economic and political pressures to accommodate massive human visitation and attendant infrastructure. An unintended consequence is the strong modulation of the three-way interaction involving people, predators and prey, a dynamic that questions the extent to which animal distributions and interactions are independent of subtle human influences. Here, I capitalize on the remarkable 9-day synchronicity in which 90% of moose neonates in the Yellowstone Ecosystem are born, to demonstrate a substantive change in how prey avoid predators; birth sites shift away from traffic-averse brown bears and towards paved roads. The decade-long modification was associated with carnivore recolonization, but neither mothers in bear-free areas nor non-parous females altered patterns of landscape use. These findings offer rigorous support that mammals use humans to shield against carnivores and raise the possibility that redistribution has occurred in other mammalian taxa due to human presence in ways we have yet to anticipate. To interpret ecologically functioning systems within parks, we must now also account for indirect anthropogenic effects on species distributions and behaviour.
Keywords: parks, predator–prey, moose, bears, fear
1. Introduction
Among the most ubiquitous recent impacts on vertebrate predator–prey dynamics are the global dissemination and explosive growth of humans in all but high Arctic landscapes (Woodroffe et al. 2005). As a consequence, the strength of interaction that once involved only native prey and native predator is now modulated by a complex, three-way community-level interaction involving people, predators and prey. In 1910, Scottish-born naturalist John Muir intimated as much: ‘Most of the animals seen today were on the Athi Plains (Kenya) and have learned that the nearer the railroad the safer they are from the attack of either men or lions’ (Branch 2001). The heightened pace of modern landscape change and overlay of human infrastructure raises fundamental questions about the extent to which we believe animal distributions and interactions are independent of even subtle human influences, including their behaviour (Blumstein 2006).
For example, in 1805, Lewis and Clark indicated that bison, elk and pronghorn were at vastly greater densities in areas of warring Native Americans (Martin & Szuter 1999), and that dangerous zones like Korea's demiliterized zone (DMZ) enhance biological diversity (Kim 1997). Nevertheless, support for the tenet that prey shield against the risk of predation by capitalization of such areas is complicated in two principle ways: (i) remnant populations may simply reflect differential harvest outside an area rather than relocation to within and (ii) spatial redistribution due to predator avoidance is confounded by alternative ecological opportunities. Consequently, recent and widely touted changes like geese using urban parks or coyotes shifting to suburbs cannot reasonably be interpreted as adaptive buffering against danger as the economics of accessible food—grass on golf courses or unwary poodles—has been altered (Beckmann & Berger 2003). A lack of simultaneous information on both the pace of prey redistribution and the intensity of responsible forces across changing landscapes renders putative cases for an ‘adaptive buffering hypothesis’ as anecdotal.
Many of the world's remaining savannah, tropical and temperate sanctuaries are under increasing human pressure (Sinclair & Arcese 1995; Brashares et al. 2001). In the USA alone, more than 400 million people visit national parks, yet little is known about indirect human impacts on refuges that often serve as references to understand functional ecosystems. Here using a 10-yr dataset, I demonstrate a novel response by which a large asocial herbivore (moose, Alces alces) develops de facto protection against its major predator of neonates (brown bears, Ursus arctos) using human infrastructure as a shield.
2. Material and methods
Each year between 1995 and 2004, my team and I focused on the timing and distribution of births for 18–25 individually marked female moose within and beyond Grand Teton National Park (GTNP) in the Yellowstone Ecosystem, USA (figure 1a). Pregnancy was initially diagnosed for 192 females based on pregnancy-specific protein B or non-invasive monitoring of faecal progestagen (Berger et al. 1999; Roffe et al. 2001). Prenatal loss characterized approximately 10% of the total pregnancies as evidenced by the proportion of births. Linear distances of females to paved roads (figures 1b and 2) were mapped or estimated by rangefinder or GPS and data subsequently log transformed to meet assumptions of normality (see electronic supplementary material).
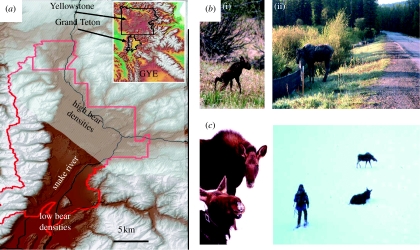
Figure 1.
(a) Location of Grand Teton National Park (red outline) within (inset) Greater Yellowstone Ecosystem (GYE), paved roads (black lines) and relative concentrations of brown bears (high density, stippled overlay). (b) (i) Newborn and (ii) calf and mother with highway and drainage fence. (c) Eight-month-old calf remains with immobile mother during recollaring.
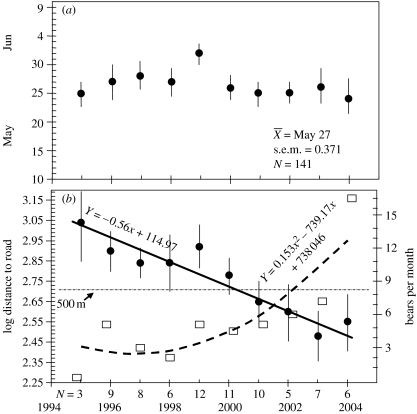
Figure 2.
(a) Dates (±1 s.d.) of 90% most clumped moose births. (b) Relationships between birth sites (log median distance) and a paved road on the date of birth (r2=0.73; p<0001) in relationship to expanding brown bears (r2=0.814; p<0001). Circles, log distance to road; squares, bears per month. N is the number of females giving birth in high-density brown bear area.
Brown bears exert strong selection, accounting for up to 90% of mortality on moose neonates (Testa 2004). Although we previously documented bear recolonization with radio-telemetry at a coarse scale (Pyare et al. 2004), I indexed bears at a finer scale using the number of new tracks and sightings as a function of field days (figure 2). The resulting nuanced spatial and temporal disparity created the experiment necessary to examine retrospectively potential changes in moose land use. One continuous and three discrete covariates were explored in a multivariate model for potential influences on female distances to paved roads: (i) time (year), (ii) pregnancy class, (iii) bear presence and (iv) migratory status, the last because some females moved to areas well beyond roads (further details in the electronic supplementary material).
3. Results
If predation on juveniles is a major force guiding female distribution, then only mothers should site births closer to areas unfrequented by bears. If this be the case, two additional corollaries must simultaneously prove true; non-pregnant females and those experiencing in utero loss fail to change land-use patterns, and will not show a similar degree of reliance on humans to buffer against predators. Since births were remarkably synchronous with 90% (n=141) falling within 9 days (figure 2a), birth site locations were contrasted to areas used by non-reproductive females (n=51) during the same period with respect to distance to roads. If the spectre of predation on neonates drives female distribution, then only mothers sympatric with bears should relocate birth sites to putatively safer regions like roads; other categories of females should not. Regression analyses (F3,188=59.903; p<0.0001; table S1 in the electronic supplementary material) demonstrate that, independently of bear distribution, non-mothers failed to change, nor did mothers in areas absent of bears. With bears, however, mothers differed in three principal ways: (i) the distance between birth sites and roads narrowed over time (figure 2b), (ii) as bear density increased, maternal distances to roads decreased, a change inversely related to the pace of bear recolonization (p<0.012) and (iii) the shift averaged 122 m yr−1 (figure 2b). Given that brown bears avoid areas within approximately 500 m of roads in Yellowstone and elsewhere (Mattson 1990; Mace 1996), mothers have apparently buffered against predation on offspring using roadside corridors (electronic supplementary material).
4. Discussion
These results suggest that bear recolonization has been a central driver of the redistribution of parous moose. Two alternative possibilities are not supported. First, the costs of lactation might induce mothers to access minerals by movement towards highways. However, there is no a priori reason to expect an incremental shift per year across the decade. Moreover, salt and other minerals are not used on Wyoming roads. Second, although brown bears accounted for 14% of the total adult mortality in GTNP (n=51) and wolf predation was less (2%), the home ranges of parturient moose and wolves did not overlap throughout 2004 (US Fish & Wildlife Service 2005, http://westerngraywolf.fws.gov/annualreports.htm). It is also possible that bear presence causes more stress or more movement such that pregnant moose experience greater in utero loss. This supposition finds little support. Out of 15 detected prenatal losses of the overall sample of 192, differences were not evident between areas with and lacking in brown bears (z=0.568, p<0.60).
Among issues salient to understand redistribution, two stand out: (i) putative mechanisms and (ii) the extent to which such behaviour characterizes other mammalian taxa. Mechanism(s) that promoted the distancing of moose from predators are not totally clear but three sources suggest involvement of maternal experience. Mothers (i) shift to new birth sites the year following bear predation but not when calves survive (Testa et al. 1998), (ii) are differentially sensitive to brown bear odours (Berger et al. 2001) and (iii) who lose offspring are approximately 8× more vigilant after sensing bear scats (Pyare & Berger 2003).
Furthermore, to assess whether offspring apparently benefit from maternal experience, I tested for concordant behaviour between mother and young by assessing responses to human predation in areas beyond the park (hunting permitted) and within (hunting prohibited). The possibility of immediate maternal effects on juvenile behaviour was discounted because mothers were immobilized for the placement of radio-collars by darting (hence, akin to human hunting) with all dyads approached on foot during winter (figure 1b,c). During 39 handling events, offspring remained with tranquilized mothers during 97% of the procedures in GTNP, but with only 25% beyond protected boundaries (Gadj,1,37=29.37; p<0.001). Not surprisingly, even with mothers anaesthetized, offspring failed to flee.
While we are uncertain whether offspring will adopt the road-induced birth tact of mothers, observations among diverse mammalian taxa suggest analogous use of human infrastructure to buffer against danger including primates, rodents, ungulates and carnivores (table 1). The most convincing evidence, however, stems not from avoidance of native carnivores but from systems with human hunters—elephants evading poachers and red deer avoiding archers.
Table 1.
Summary of mammalian taxonomic diversity in potentiala use of human infrastructure to buffer against predation.
| prey–predator system | location | human construct | inference |
|---|---|---|---|
| vervet monkey–leopard (Isbell 1990) | Amboseli park, Kenya | ranger station | apredation dampened by human presence |
| gelada baboon–spotted hyena (Kummer 1995) | Ahmar mountains, Ethiopia | researcher presence | apeople-averse predators |
| marmot–badger (Armitage 2004) | Rocky mountains, USA | fence post | aenhanced vigilance towards predators by climbing |
| axis deer–tiger (Sunquist & Sunquist 1989) | Chitwan, Nepal | tourist centre | aavoidance of area by predators |
| elephants–poachers (Foley et al. 2001) | Tarangire park, Tanzania | protected park | movement to park |
| red deer–big game hunters (Connor et al. 2001) | Rocky mountains, USA | hunt-free zones | movement to ranchers, changed migration routes |
Additional evidence needed to substantiate suggested pattern.
The redistribution of moose mothers may, however, represent a fleeting phenomenon. Brown bears are adaptable carnivores and exploit many foods. Unlike the Tetons, where these carnivores were absent for 60 years (Berger et al. 2001), their presence has been continuous in Alaska and the northern Rockies, where moose show no propensity for births near roads (Langley & Pletscher 1994; Bowyer et al. 1999), and bears are not road averse (Albert & Bowyer 1991; Yost & Wright 2001). If GTNP moose respond to bears as they do elsewhere, the attractiveness of roadsides will fade as bear reoccupancy continues and a landscape of fear envelopes the entire ecosystem (electronic supplementary material).
Today's protected areas attract more people and a coincident desire for additional constructs, such as bike paths, lodges and roads. A failure to understand how the veil of anthropogenic actions not only affects carnivores themselves, but also governs species interactions, will negate the possibility of using parks as repositories to gauge ecological change. Prey buffering by use of humans for antipredatory shields is only one example. Given the intensification of economic and political pressures beyond parks that conspire to increase revenues by attracting more human visitation to them, it is incumbent upon us to discover other consequences resulting from our presence.
Acknowledgments
Grants from the National Science Foundation and the Charles Engelhard Foundation, and permissions from the National Park Service (Grand Teton) and the Wyoming Department of Game and Fish enabled this work. I thank S. Cain, D. Craighead, C. Cunningham, K. Murray-Berger, M. Nordell, S. Pyare, M. Reid, N. Weber and R. Wulff for their fieldwork, and J. Beckmann, M. Conner, J. S. Brown, C. Foley, J. Hilty, D. Mattson, K. Murray-Berger and T. O'Brien for their insights. The handling of animals in this study was done under direct veterinary supervision and all ethical standards of the Wildlife Conservation Society.
Supplementary Material
Supplementary methods; results; discussion
References
- Albert D.M, Bowyer R.T. Factors related to grizzly bear–human interactions in Denali national park. Wildl. Soc. Bull. 1991;19:339–349. [Google Scholar]
- Armitage K.B. Badger predation on yellow-bellied marmots. Am. Midl. Nat. 2004;151:378–387. doi:10.1674/0003-0031(2004)151[0378:BPOYM]2.0.CO;2 [Google Scholar]
- Beckman J.P, Berger J. Rapid ecological and behavioural changes in carnivores: the responses of black bears (Ursus americanus) to altered food. J. Zool. 2003;261:207–212. doi:10.1017/S0952836903004126 [Google Scholar]
- Berger J, Testa J.W, Roffe T, Montfort S.L. Conservation endocrinology: a noninvasive tool to understand relationships between carnivore colonization and ecological carrying capacity. Conserv. Biol. 1999;13:980–989. doi:10.1046/j.1523-1739.1999.98521.x [Google Scholar]
- Berger J, Swenson J.E, Persson I.-L. Re-colonizing carnivores and naive prey; conservation lessons from Pleistocene extinctions. Science. 2001;291:1036–1039. doi: 10.1126/science.1056466. doi:10.1126/science.1056466 [DOI] [PubMed] [Google Scholar]
- Blumstein D.T. Developing an evolutionary ecology of fear: how life history and natural history affect disturbance tolerance in birds. Anim. Behav. 2006;71:389–399. doi:10.1016/j.anbehav.2005.05.010 [Google Scholar]
- Bowyer R.T, van Ballenberghe V, Kie J.G, Maier J.A.K. Birth-site selection by Alaskan moose: maternal strategies for coping with a risky environment. J. Mammal. 1999;80:1070–1083. doi:10.2307/1383161 [Google Scholar]
- Branch M.P, editor. John Muir's last journey: unpublished journals and selected correspondence. Island Press; Covello, CA: 2001. [Google Scholar]
- Brashares J.S, Arcese P, Sam M.K. Human demography and reserve size predict wildlife extinction in west Africa. Proc. R. Soc. B. 2001;268:2473–2478. doi: 10.1098/rspb.2001.1815. doi:10.1098/rspb.2001.1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M.M, White G.C, Freddy D.J. Elk movement in response to early-season hunting in northwest Colorado. J. Wildl. Manage. 2001;6:926–940. [Google Scholar]
- Foley C.A.H, Papageorge S, Wasser S.K. Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conserv. Biol. 2001;15:1134–1142. doi:10.1046/j.1523-1739.2001.0150041134.x [Google Scholar]
- Isbell L.A. Sudden short-term increase in mortality of vervet monkeys (Cercopithecus aethiops) due to leopard predation in Amboseli national park, Kenya. Am. J. Primatol. 1990;21:41–52. doi: 10.1002/ajp.1350210105. doi:10.1002/ajp.1350210105 [DOI] [PubMed] [Google Scholar]
- Kim K.C. Preserving biodiversity in Korea's demilitarized zome. Science. 1997;278:242–243. doi:10.1126/science.278.5336.242 [Google Scholar]
- Kummer H. Princeton University Press; Princeton, NJ: 1995. Quest of the sacred baboon. [Google Scholar]
- Langley M.A, Pletscher D.H. Calving areas of moose in northwestern Montana and southeastern British Columbia. Alces. 1994;30:127–136. [Google Scholar]
- Mace R.D. Relationships among grizzly bears, roads and habitat in the Swan mountains, Montana. J. Appl. Ecol. 1996;33:1395–1404. doi:10.2307/2404779 [Google Scholar]
- Martin P.S, Szuter C.R. War zones and game sinks in Lewis and Clark's west. Conserv. Biol. 1999;13:36–45. doi:10.1046/j.1523-1739.1999.97417.x [Google Scholar]
- Mattson D.J. Human impacts on bear habitat use. Int. Conf. Bear Res. Manage. 1990;8:35–56. [Google Scholar]
- Pyare S, Berger J. Beyond demography and de-listing: ecological recovery for Yellowstone's grizzly bears and wolves. Biol. Conserv. 2003;113:63–73. doi:10.1016/S0006-3207(02)00350-6 [Google Scholar]
- Pyare S, Cain S, Moody D, Schwartz C, Berger J. Grizzly bears in the Yellowstone ecosystem; loss and re-colonization rates during a century of change. Anim. Conserv. 2004;7:71–78. doi:10.1017/S1367943003001203 [Google Scholar]
- Roffe T.J, Coffin K, Berger J. Survival and immobilizing moose with carfentanil and xylazine. Wildl. Soc. Bull. 2001;29:1140–1146. [Google Scholar]
- Sinclair A.R.E, Arcese P, editors. Serengeti 2; dynamics, management, and conservation of an ecosystem. University of Chicago Press; Chicago, IL: 1995. [Google Scholar]
- Sunquist F, Sunquist M.E. University of Chicago Press; Chicago, IL: 1989. Tiger moon: tracking the great cats in Nepal. [Google Scholar]
- Testa J.W. Interaction of top-down and bottom-up life-history trade-offs in moose (Alces alces) Ecology. 2004;85:1453–1459. doi:10.1890/02-0672 [Google Scholar]
- Testa J.W, Becker E.F, Lee G.R. Movements of female moose in relation to birth and death of calves. Alces. 1998;36:155–162. [Google Scholar]
- Woodroffe R, Thirgood S, Rabinowitz A. Cambridge University Press; Cambridge, UK: 2005. People and wildlife: conflict or coexistence? [Google Scholar]
- Yost A.C, Wright R.G. Moose, caribou, and grizzly bear distribution in relation to road traffic in Denali national park. Arctic. 2001;54:41–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods; results; discussion