Abstract
Bumble-bee declines across Europe have been linked to loss of habitat and forage availability due to agricultural intensification. These declines may have severe ecological and commercial consequences since bumble-bees pollinate a range of wildflowers and crops. In England, attempts are being made to reintroduce forage resources through agri-environment schemes, yet there are few data on how the area of forage, or the landscape context in which it is provided, affects their success. We investigated the effects of sown forage patches on bumble-bees across sites varying in landscape characteristics. Bumble-bee densities were higher on sown patches compared with control habitats but did not vary with patch size, i.e. total forager numbers were proportional to patch area. Importantly, the relative response to sown forage patches varied widely across a landscape gradient such that their impact in terms of attracting foraging bumble-bees was greatest where the proportion of arable land was highest.
Keywords: Bombus, forage plants, pollination
1. Introduction
Bumble-bees (Bombus spp.) are important pollinators of a large number of native plant species and some crops (Corbet et al. 1991). Recent declines in their abundance in Europe have been linked to habitat loss and alteration resulting from intensified agriculture (Goulson et al. 2005). In particular, intensified crop management and reductions in mixed farming have led to the loss, or botanical simplification, of both semi-natural habitats (e.g. flower-rich meadows) and linear habitats (e.g. species-rich hedgerows and margins; Robinson & Sutherland 2002). This has led to a widespread decline in the quality and abundance of forage resources and nesting habitats for bumble-bees (Carvell et al. 2006).
Reduced pollination services can have detrimental effects on the dynamics and persistence of plant species and communities (Fontaine et al. 2006). While diverse pollinator assemblages may be important to the maintenance of these services, bumble-bees may be able to compensate for the losses in other pollinator groups. This makes them key species in some agro-ecosystems (Kremen et al. 2002, 2007).
The European Union has recognized the need to counteract the negative effects of modern agriculture on the environment, and has introduced agri-environment schemes whereby farmers are paid to manage their land for the benefit of particular habitats and species. England has recently adopted the Environmental Stewardship scheme (www.defra.gov.uk/erdp/schemes/es/default.htm). This includes specific options targeted at pollinators, aiming to enhance the supply of pollen and nectar sources by sowing flower mixtures at field margins. These mixtures can significantly enhance the local density and diversity of foraging bumble-bees on arable land (Carvell et al. 2004), yet their effects have not been studied with respect to the area of forage or landscape context in which they are provided.
Landscape context, especially the availability of semi-natural habitats, has been recognized as important to bumble-bees and may interact with farming systems to determine the local community structure (Tscharntke et al. 2005). However, recent work questions the benefit of semi-natural habitat to bumble-bee communities within agricultural landscapes (Westphal et al. 2003; Kleijn et al. 2006). These studies have considered neither the targeted agri-environment scheme options for pollinators being implemented in the UK nor the response of bumble-bees to introduced forage resources relative to resources elsewhere in the landscape. Here, we investigate the responses of bumble-bees to habitat creation in different agricultural landscapes. We test if sown forage patches have a positive effect on forager densities and if the effect is influenced by landscape context.
2. Material and methods
We selected eight sites across central and eastern England that represented typical land use for their locations but varied widely in landscape characteristics (table 1). We randomly allocated four treatments to each site: three forage patches of 0.25, 0.5 and 1 ha sown with a mixture of 20% legumes (Trifolium pratense, Trifolium hybridum and Lotus corniculatus) and 80% fine-leaved grasses (Festuca rubra, Poa pratensis and Cynosurus cristatus), and a control patch representing typical non-crop vegetation for the site. The loss of legumes is suggested as a major driver of declines of longer-tongued bumble-bees (Goulson et al. 2005); hence we expected that these bumble-bee species would be most attracted to the sown patches. Each patch was separated by approximately 3 km (mean=2.99±0.23 km, s.e.) to reduce bumble-bee dispersal between them (Knight et al. 2005). Patches were established between autumn 2003 and spring 2004.
Table 1.
Landscape characteristics averaged across all patches (n=4) within sites. ANOVA revealed no significant differences between patch area characteristics within a site for each landscape type (p>0.05).
| site | arablea % (s.e.) | arable range | grassb % (s.e.) | grass range | woodland % (s.e.) | woodland range | urban % (s.e.) | urban range |
|---|---|---|---|---|---|---|---|---|
| 1 | 89.7 (2.1) | 84.6–93.4 | 8.0 (2.6) | 2.9–13.5 | 0.2 (0.2) | 0–0.6 | 1.8 (0.7) | 0.2–3.6 |
| 2 | 81.2 (4.7) | 75.2–95.2 | 15.2 (4.2) | 3–21.5 | 1.7 (0.9) | 0–4 | 1.8 (0.6) | 0.9–3.5 |
| 3 | 73.9 (2.7) | 66.4–79.4 | 16.6 (5.4) | 5.2–27.9 | 7.3 (4.2) | 0.6–19.7 | 1.9 (1.4) | 0–6.1 |
| 4 | 71.6 (4.0) | 63.3–81.0 | 22.3 (4.9) | 15.0–36.2 | 4.2 (2.3) | 0–8.8 | 0.6 (0.5) | 0–2.0 |
| 5 | 67.6 (4.5) | 59.2–79.4 | 18.4 (2.6) | 13.1–25.6 | 8.9 (2.1) | 4.7–14 | 4.7 (3.1) | 0–13.1 |
| 6 | 34.8 (5.5) | 19.9–46.3 | 30.7 (7.5) | 13.9–50.2 | 20.5 (3.2) | 13.0–28.2 | 11.4 (3.0) | 4.4–16.7 |
| 7 | 26.9 (2.7) | 22.7–34.7 | 57.9 (6.4) | 39.5–68.4 | 12.7 (3.8) | 7.6–23.8 | 2.2 (0.7) | 0.8–3.9 |
| 8 | 26.5 (3.3) | 17.4–33.5 | 55.8 (3.1) | 49.3–64.4 | 10.8 (2.8) | 6.8–19.2 | 3.3 (0.7) | 2.4–5.2 |
Includes cereals and mass flowering crops, for example, rape oilseed, field bean, potato.
Includes improved and unimproved grassland, set-aside.
A circular sampling zone around each treatment patch (n=32) was extended to a radius of 1 km (314 ha) to reflect the scale at which the longer-tongued bumble-bees forage (Knight et al. 2005). Landscape characteristics for each zone were obtained from the Land Cover Map 2000 (www.ceh.ac.uk/data/lcm), a computer-classified land cover dataset derived from satellite-based multispectral scanners. We used the proportion of arable cropped fields as an indicator of landscape context because it was negatively correlated with the proportions of grassland, woodland (p<0.001) and urban cover (p<0.05).
Bumble-bee activity was recorded monthly from May to September 2005 (five occasions) when all the patches were successfully established and were flowering. Foraging bumble-bees were counted along two fixed 2 m×100 m transects in the centre of each treatment patch, and the plant species on which they were foraging were noted (Banaszak 1980). In small or irregularly shaped patches we used U-shaped transects to cover an equivalent area (e.g. four 50×2 m transects). All social Bombus species, cuckoo bees (subgenus Psithyrus) and honeybees (Apis mellifera) were recorded. For analysis, we grouped social Bombus species into colour groups (after Fussell & Corbet 1992, but with melanic Bombus ruderatus as a separate group), tongue length groups (long, greater than 8.5 mm; short, less than 8.5 mm) and diet breadth groups (defined using Simpson's diversity index, D, based on our flower visitation data; mean=3.8, narrow: D<3.8; broad: D>3.8; table 2). Transects were carried out between 10.00 and 17.30 during dry weather when ambient temperature was above 13°C with at least 60% clear sky, or 17°C under any sky condition.
Table 2.
Bumble-bee forager density on control and forage patches and ANOVA tests for differences between (i) control and forage patches and (ii) sown forage patches. S, short-tongued; L, long-tongued; BD, broad diet; ND, narrow diet. *p<0.05, ***p<0.001.
| bumble-bee species or group | treatment mean (±s.e.) | p-value of contrast | ||
|---|---|---|---|---|
| control | forage patch | control versus forage patches (1 d.f.) | between forage patches (2 d.f.) | |
| Bombus spp. | 1.78 (0.70) | 25.65 (4.30) | <0.001*** | 0.91 |
| B. hortorum (L, ND) | 0.18 (0.11) | 2.05 (0.43) | <0.001*** | 0.25 |
| B. lapidarius (S, ND) | 0.83 (0.32) | 13.26 (2.25) | <0.001*** | 0.96 |
| B. pascuorum (L, ND) | 0.39 (0.21) | 8.75 (1.89) | <0.001*** | 0.62 |
| B. pratorum (S, BD) | 0.05 (0.05) | 0.03 (0.01) | 0.86 | 0.66 |
| B. ruderatus (L, ND) | 0.04 (0.04) | 0.09 (0.05) | 0.50 | 0.93 |
| B. terrestris agg (S, BD) | 0.30 (0.12) | 1.48 (0.35) | 0.016* | 0.22 |
| short-tongued Bombus spp. | 1.18 (0.43) | 14.77 (2.47) | <0.001*** | 0.99 |
| long-tongued Bombus spp. | 0.60 (0.34) | 10.88 (2.01) | <0.001*** | 0.92 |
| broad diet Bombus spp. | 0.35 (0.16) | 1.50 (0.36) | 0.013* | 0.25 |
| narrow diet Bombus spp. | 1.43 (0.56) | 24.14 (4.07) | <0.001*** | 0.87 |
| Psithyrus spp. | 0.14 (0.11) | 0.30 (0.11) | 0.22 | 0.99 |
| Apis mellifera | 0.40 (0.33) | 0.92 (0.28) | 0.077 | 0.14 |
Forage availability on every visit was measured by identifying all flowering dicotyledonous species and scoring their flower abundance in each 2×10 m transect section within the following ranges: 1–5; 6–25; 26–200; 201–1000; 1001–4999; and 5000+ flower units (defined as a single flower or an umbel, spike or capitulum on multi-flowered stems). For analysis, flower abundance was expressed as the median value for each range, giving an estimate of the number of flowering units on each sampling visit.
The effects of patches on bee density were tested using a randomized block ANOVA on log-transformed mean per transect (across all visits) with site and treatment as factors, and contrasts for effects of treatment type (control versus sown) and patch size. The differences in flower density (mean number of flowers per transect, per sampling visit) between treatment patches across the landscape sectors, were tested by ANOVA, and contrasts. The relationship between bee density and percentage of arable across sites was compared between control and sown forage patches allowing for a random site effect using residual maximum likelihood (REML).
3. Results
We observed 6602 bees including 9 social bumble-bee species and at least 3 cuckoo bee species. The most common species groups were: Bombus lapidarius (+Bombus ruderarius) 45%; Bombus pascuorum (+Bombus muscorum/humilis) 31%; Bombus terrestris agg. (+Bombus lucorum) 8%; Bombus hortorum 8%; Bombus ruderatus 0.5%; and Bombus pratorum 0.4%.
Bumble-bee density was significantly higher on the sown forage treatments than on the control patches (table 2) but did not differ significantly with sown patch size for any species (table 2), suggesting that total bumble-bee numbers increased in proportion to patch area. Individual bumble-bee species or groups showed variable responses to the sown patches. As expected, the strongest positive response was from the longer-tongued species (Goulson et al. 2005), with B. lapidarius and B. pascuorum, the most commonly recorded, 15–35 times more abundant on the sown forage patches than control areas. Psithyrus spp. and A. mellifera did not show a significant response to sown forage patches. The mean density of visited flowers (defined as species that bumble-bees visited at least once in the study) was significantly higher on the sown forage patches than the control (F1,23=40.9, p<0.001) but did not differ significantly between sown forage patches (F2,14=1.01, p=0.39) within or between sites (F7,14=1.2, p=0.37).
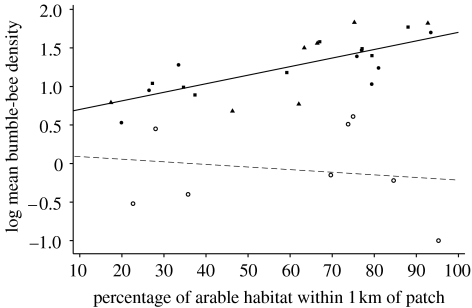
The effect of landscape context was highly significant in determining the response of bumble-bee density to forage patches but did not affect honeybee density which declined in both forage patches and control areas with increasing proportion of arable land (table 3). In general, significantly more bumble-bees per transect were found on forage patches, in landscapes, in which the proportion of arable land was highest. Importantly, the density of narrow diet and longer-tongued species on control patches decreased with increasing proportion of arable land in the habitat surrounding the patches (figure 1). This suggests that non-crop habitats surrounding the patches were poorer for bumble-bees as the proportion of arable land increased (e.g. control patch log legume cover r=−0.72, p=0.043). There was no significant correlation between honeybee and bumble-bee abundance suggesting little or no competition for forage resources (r=−0.296, p=0.106). Finally, there was a strong positive correlation between the mean estimated number of flowers of all bumble-bee forage plant species and the mean total number of bumble-bees per patch (total Bombus spp.=0.61+0.0022×total forage flowers, r=0.74, p<0.001).
Table 3.
Estimated slopes relating bumble-bee density to percentage of arable land across sites for control and sown forage patches, with Wald test for differences in slopes. *p<0.05, **p<0.01.
| control | sown | comparison of slopes | ||||||
|---|---|---|---|---|---|---|---|---|
| bumble-bee species or group | intercept | slope | s.e. | intercept | slope | s.e. | Wald | p |
| Bombus spp. | 0.14 | −0.0026 | 0.0051 | 0.61 | 0.011 | 0.037 | 6.18 | 0.013* |
| B. hortorum | −0.76 | 0.0007 | 0.0055 | −0.48 | 0.010 | 0.0045 | 3.62 | 0.057 |
| B. lapidarius | −0.20 | −0.0011 | 0.0051 | 0.44 | 0.009 | 0.0040 | 3.86 | 0.049* |
| B. pascuorum | −0.59 | 0.0001 | 0.0059 | −0.15 | 0.014 | 0.0038 | 4.22 | 0.04* |
| B. pratorum | −1.02 | 0.0018 | 0.0026 | −1.00 | 0.001 | 0.0018 | 0.02 | 0.88 |
| B. ruderatus | −1.06 | 0.0023 | 0.0036 | −1.16 | 0.005 | 0.0029 | 0.63 | 0.43 |
| B. terrestris agg | −0.67 | 0.0022 | 0.0063 | −0.58 | 0.009 | 0.0044 | 0.9 | 0.34 |
| broad diet Bombus spp. | −0.68 | 0.0026 | 0.0062 | −0.57 | 0.009 | 0.0043 | 0.87 | 0.35 |
| narrow diet Bombus spp. | 0.08 | −0.0028 | 0.0050 | 0.58 | 0.011 | 0.0037 | 6.82 | 0.009** |
| short-tongued Bombus spp. | −0.05 | −0.0015 | 0.0051 | 0.48 | 0.009 | 0.0039 | 4.28 | 0.039* |
| long-tongued Bombus spp. | −0.33 | −0.0019 | 0.0051 | 0.01 | 0.014 | 0.0035 | 7.34 | 0.007** |
| Psithyrus spp. | −0.53 | −0.0047 | 0.0052 | −1.16 | 0.009 | 0.0044 | 7.93 | 0.005** |
| Apis mellifera | −0.46 | −0.0033 | 0.0066 | 0.47 | −0.014 | 0.005 | 2.17 | 0.141 |
Figure 1.
Percentage of arable habitat in relation to mean density (log) of narrow diet bumble-bees on transects (2×100 m) on sown (filled triangle, 0.25 ha; filled square, 0.5 ha; filled circle, 1.0 ha) and control (open circle) patches. Fitted line equations, control y=0.077−0.0028x; sown y=0.58+0.011x.
4. Discussion
Our analyses suggest that the higher densities of foraging bumble-bees attracted to sown forage patches did not vary with patch size but did with landscape context. If at the start of the experiment, patches were isolated (i.e. no shared foragers), bumble-bee colonies were randomly distributed within a landscape and bees foraged only on patches, we would expect forager density to decline with increasing patch size. An absence of this trend is consistent with several alternative explanations. They are: (i) an ideal free distribution; this would occur if patches were not truly isolated and bumble-bees travelled between them, but seems unlikely given the limited foraging ranges described for several species, for example, B. pascuorum, B. lapidarius 449–450 m (Knight et al. 2005), (ii) higher colony growth rates near larger patches (more total forage) led to an increase in foragers visiting larger patches, offsetting expected decreases in density, and (iii) not all foragers within an area visited a patch, but larger patches were encountered more often, thus the proportion of foragers visiting a patch scales with its size.
In common with previous work we found higher numbers of bees on sown forage patches in areas with high levels of agriculture. Westphal et al. (2003) concluded that the abundance of mass flowering crops (MFCs), rather than that of semi-natural habitats, was an effective determinant of bumble-bee forager density on flowering patches. By contrast, our data suggest that the response to introduced forage patches was driven by a lack of forage resources in the surrounding habitats typified by the control patches (MFCs had flowered earlier in all landscapes). Across our landscape gradient, increasing arable area led to a reduction in both the quantity and the quality of semi-natural forage resources for bumble-bees. Thus, sown patches were relatively more exploited where the availability of resources from semi-natural habitats was limited. The mobility of bumble-bees means that they appear capable of readily locating and exploiting high quality forage patches, at least within the scale at which we sampled.
Although the Bombus species assemblages differed across sites, many bumble-bees, especially the longer-tongued and narrow diet species, showed a positive response to the sown mixture of legume species. Bumble-bees often show strong preferences for certain flowers or plant families (Heinrich 1976). The decline in our sown legume species in the UK countryside may be a principal cause of rarity and decline in some bumble-bees (Goulson et al. 2005). Our results suggest that restoring forage resources through agri-environment schemes can enhance bumble-bee densities and attract large numbers of foraging bumble-bees, especially in intensively managed agricultural landscapes. While our results focus on densities of individuals, it is important to realize that this may not reflect impacts on populations per se. This requires more direct measurements of either colony density or colony performance, and these form the basis of our ongoing work.
Acknowledgments
We thank W. Jordan for his comments and S. and L. Hulmes, P. Nuttall, A. Martin, C. Shortall and J. Swain for fieldwork. This research was funded by Defra and Natural England.
References
- Banaszak J. Studies on methods of censusing the numbers of bees (Hymenoptera: Apoidea) Pol. Ecol. Studies. 1980;6:355–366. [Google Scholar]
- Carvell C, Meek W, Pywell R, Nowakowski M. The response of foraging bumble-bees to successional change in newly created arable field margins. Biol. Conserv. 2004;118:327–339. doi:10.1016/j.biocon.2003.09.012 [Google Scholar]
- Carvell C, Roy D, Smart S, Pywell R, Preston C, Goulson D. Declines in forage availability for bumble-bees at a national scale. Biol. Conserv. 2006;132:481–489. doi:10.1016/j.biocon.2006.05.008 [Google Scholar]
- Corbet S.A, Williams I.H, Osborne J.L. Bees and the pollination of crops and wild flowers in the European Community. Bee World. 1991;72:47–59. [Google Scholar]
- Fontaine C, Dajoz I, Meriguet J, Loreau M. Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol. 2006;4:129–135. doi: 10.1371/journal.pbio.0040001. doi:10.1371/journal.pbio.0040001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussell M, Corbet S.A. Flower usage by bumble-bees: a basis for forage plant management. J. Appl. Ecol. 1992;29:451–465. doi:10.2307/2404513 [Google Scholar]
- Goulson D, Hanley M, Darvill B, Ellis J, Knight M.E. Causes of rarity in bumble-bees. Biol. Conserv. 2005;122:1–8. doi:10.1016/j.biocon.2004.06.017 [Google Scholar]
- Heinrich B. The foraging specializations of individual bumble-bees. Ecol. Monogr. 1976;46:105–128. doi:10.2307/1942246 [Google Scholar]
- Kleijn D, et al. Mixed biodiversity benefits of agri-environment schemes in five European countries. Ecol. Lett. 2006;9:243–254. doi: 10.1111/j.1461-0248.2005.00869.x. doi:10.1111/j.1461-0248.2005.00869.x [DOI] [PubMed] [Google Scholar]
- Knight M.E, Bishop S, Martin A.P, Osborne J.L, Hale R.J, Sanderson R.A, Goulson D. An interspecific comparison of foraging range and nest density of four bumble-bee (Bombus) species. Mol. Ecol. 2005;14:1811–1820. doi: 10.1111/j.1365-294X.2005.02540.x. doi:10.1111/j.1365-294X.2005.02540.x [DOI] [PubMed] [Google Scholar]
- Kremen C, Williams N, Thorp R. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl Acad. Sci. USA. 2002;99:16 812–16 816. doi: 10.1073/pnas.262413599. doi:10.1073/pnas.262413599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C, et al. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol. Lett. 2007;10:299–314. doi: 10.1111/j.1461-0248.2007.01018.x. doi:10.1111/j.1461-0248.2007.01018.x [DOI] [PubMed] [Google Scholar]
- Robinson R.A, Sutherland W.J. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 2002;39:157–176. doi:10.1046/j.1365-2664.2002.00695.x [Google Scholar]
- Tscharntke T, Klein A.M, Kruess A, Steffan-Dewenter I, Thies C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 2005;8:857–874. doi:10.1111/j.1461-0248.2005.00782.x [Google Scholar]
- Westphal C, Steffan-Dewenter I, Tscharntke T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003;6:961–965. doi:10.1046/j.1461-0248.2003.00523.x [Google Scholar]