Abstract
Mechanistic models of animal signals posit the occurrence of biases on the part of receivers that could be potentially exploited by signallers. Such biases are most obvious when animals are confronted with exaggerated versions of signals they normally encounter. Signalling systems operating in plant–pollinator interactions are among the most highly coevolved, with plants using a variety of floral signals to attract pollinators. A number of observations suggest that pollinators preferentially visit larger floral displays although the benefit of this to either the plant or the pollinator is not always clear. We use a standard dual-choice experimental protocol to show that honeybees display a receiver bias for exaggerated size and colour contrast—two important components of floral signals—even when such signals do not indicate quality. We discuss the implications of this receiver bias for the evolution of floral displays and its possible exploitation by invading alien plants.
Keywords: receiver bias, exaggerated signals, floral displays, pattern discrimination, pollinators, honeybees
1. Introduction
The role of receiver bias is a dominant theme in current research on signals. The idea of receiver bias predicts that signal recognition systems are not adaptively designed to respond only to all appropriate stimuli and not respond to any inappropriate stimuli. Therefore, the observed response of a receiver to a given signal cannot exactly predict its reaction to a variant of the same signal or a completely novel signal. This, in turn, suggests that there is always the potential for a new signal to elicit an even higher response from the receiver than the original signal. This potential ‘hidden preference’ is not expressed and therefore not seen until the receiver is exposed to the novel signal by an invading mutant or by an experiment (as given in this paper). The existence of such biases has been most clearly supported in empirical studies of sexual selection, showing that females respond more strongly to exaggerated male traits (Basolo 1990; Ryan & Rand 1993; Wilkinson & Reillo 1994).
Arak & Enquist (1993) used a neural network model to demonstrate a possible mechanistic basis for the idea of receiver bias for exaggerated signals, using a plant–pollinator system as an example. In spite of the obvious connection of this idea to numerous observations that pollinators prefer larger floral displays, researchers have been disinclined to seriously consider factors other than a reward that might influence pollinator preference. Despite observations such as that flower size does not necessarily correlate with reward size and that naive bees show a strong preference for larger displays, pollinator preference for larger displays is still mainly attributed to different costs and benefits associated with foraging on flowers of different sizes (Harder & Cruzan 1990; Makino & Sakai 2007). We instead propose that receiver bias for exaggerated signals plays a strong role in pollinator preference for larger displays, and this can be demonstrated by isolating the signalling components of a floral display from the foraging costs and benefits associated with it. We test our hypothesis with a dual-choice experimental set-up that determines the preference of the generalist pollinator, the European honeybee Apis mellifera, for exaggerated signals in terms of size and colour contrast of a display.
2. Material and methods
(a) Experimental set-up
The experimental set-up consisted of a Y-maze apparatus (Srinivasan & Lehrer 1988) on a tabletop located at a distance of 10 m from a free foraging hive. We trained individually marked bees to fly into the Y-maze apparatus, allowing them to enter it one at a time. A bee, upon entering the decision chamber of the maze, could simultaneously view two patterns, each presented on a vertical plane at the end wall of an arm. One of the patterns, termed positive, offered a reward of 20% sugar water at its centre, dispensed by a syringe tip that was connected to a pump set at a delivery rate of 100 μl min−1 and triggered on by the landing of the bee on the tip. The other pattern, termed negative, offered no reward at the syringe tip at its centre. The bees make a choice within the decision chamber to enter one of the arms, and the first crossing of the boundary between the decision chamber and this arm was counted as a choice on the part of the bee.
(b) Training and testing
Using naive bees for each day's of training and testing, each experiment was conducted with approximately 15 bees. In order to ensure that the bees do not associate the reward with a particular arm of the maze, the position of the rewarding and non-rewarding patterns was interchanged between the two arms after each bee, on average, has made two visits. The choice of the bee was scored as correct if the bee entered the arm with the rewarded pattern. A bee was trained to learn the two patterns until its choice level for the positive pattern was significantly different from random (binomial test).
Following this, we performed a discrimination test in which the positive pattern was identical to the one during training while the alternative negative pattern was novel to the bees. During the test, each bee was rewarded four times on average, twice in each arm. After each test, we resumed training with the two original patterns and carried it out for at least 10 visits per bee before conducting another test. Training and testing related to a single experiment was repeated over subsequent days until the bees had made at least 50 test choices. The choice frequencies of all bees were pooled after testing for homogeneity (G-test), and analysed to determine whether it was significantly different from random (see the electronic supplementary material for the learning curves and choice frequencies of individual bees).
We performed three different experiments to test whether bees showed any preference for exaggerations of the two important components of floral signals: size and colour contrast. The first two experiments were designed to test the preference for a signal that varied in a single stimulus dimension, while the next tested the preference when the signal varied in two dimensions simultaneously.
3. Results
(a) Experiment 1: varying size
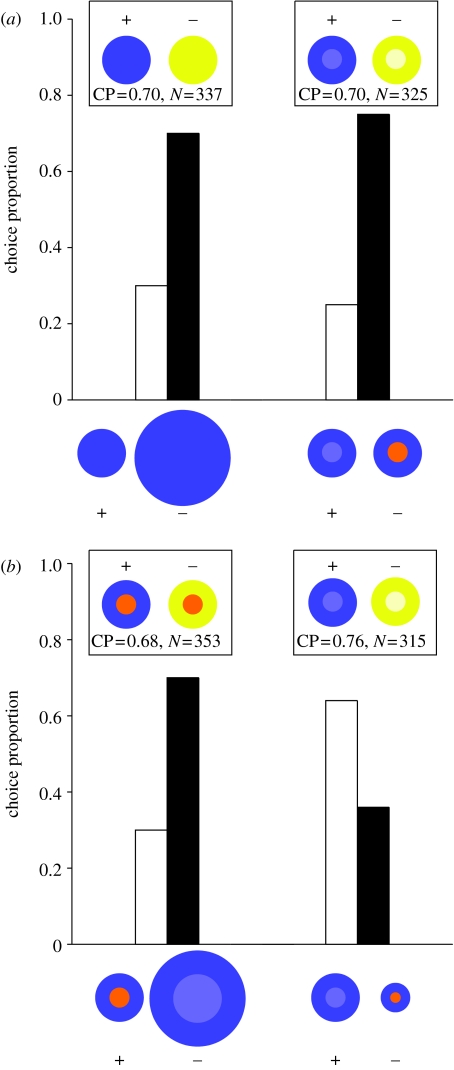
This experiment tested whether bees trained to a pattern have a higher preference for a larger size of the same pattern. In two different replicates of this experiment, we trained bees to a blue or a yellow circle of 6 cm in diameter, one of the two colours serving as positive and the other negative. We then tested their choice between the previously learned positive pattern and a novel pattern of the same colour but twice in size (12 cm in diameter). In both the replicates, the bees learned to discriminate between the positive and the negative patterns during training and significantly preferred the larger pattern during the test (blue: p=0.007, N=50 choices by 13 bees, figure 1a; yellow: p=0.013, N=72 choices by 16 bees, see electronic supplementary material).
Figure 1.
Choice frequencies showing preferences for exaggerated size and colour contrast. In (a,b), the x-axis denotes the choice offered to the trained bees during testing and the bars depict the choice frequencies obtained in favour of each pattern. The insets show the training patterns, the total number of choices and the choice frequency for the positive pattern during training. (a) Varying size and contrast individually and (b) varying size and contrast simultaneously.
(b) Experiment 2: varying contrast
This experiment tested whether bees trained to a pattern have a higher preference for a similar pattern but with a higher contrast. In two different replicates of this experiment, we trained bees to either a blue circle with a pale blue centre or a yellow circle with a pale yellow centre. The circle and the centre in each case had diameters of 6 and 3 cm, respectively. We then tested their choice between the positive pattern they learned during training and a circle of the same colour but with an orange centre. Bees learned to discriminate the positive from the negative pattern in both the replicates and significantly preferred the pattern with the orange centre during the test (blue: p<0.001, N=71 choices by 18 bees, figure 1a; yellow: p=0.007, N=62 choices by 16 bees, see electronic supplementary material).
(c) Experiment 3: varying size and contrast
In the first replicate of this experiment, we trained bees to a blue circle (6 cm in diameter) with an orange centre (3 cm in diameter) and then tested their choice between this positive pattern and a larger blue circle (12 cm in diameter) with a pale blue centre (6 cm in diameter). Bees could discriminate the positive pattern during training and significantly preferred the novel test pattern of a larger circle even though it had a lower contrast (p=0.003, N=65 choices by 16 bees; figure 1b). In the second replicate, we trained bees to a blue circle (6 cm in diameter) with a pale blue centre (3 cm in diameter) and tested their choice between this positive pattern and a blue circle (3 cm in diameter) with an orange centre (1.5 cm in diameter). Bees could discriminate the positive pattern during training and significantly preferred the original training pattern of the larger circle even though it had a lower colour contrast (p=0.036, N=59 choices by 15 bees; figure 1b).
4. Discussion
It has been documented that honeybees can discriminate objects based on signals such as size and chromatic contrast (Horridge et al. 1992; Hempel de Ibarra et al. 2002). In addition, our results demonstrate that once bees learn to associate a reward with any of these signals, they show a preference for exaggerated versions of the same signals even when the signal strength is not correlated with the reward value. The extent of this bias could be different for various components of the floral signal; in this case, size superseding the extent of contrast in the design. This supports our hypothesis that bees display a receiver bias for exaggerated signals, a bias that could arise as a non-adaptive epiphenomenon of either the neural architecture or the learning mechanism involved (ten Cate & Rowe 2007).
Floral signals for attracting pollinators could therefore be open to invasion by cheater plants that exaggerate these signals without a parallel increase in reward. It has, however, been argued that such dishonest signalling could evolve only if pollinator preference is innate and not if it is modulated via associative learning (Blarer et al. 2002). This stated dichotomy between innate and learned preferences is false and innate preferences can certainly weaken but not completely disappear through learning. Such innate biases would therefore still benefit a flower with an exaggerated signal provided the reward it offers matches the one offered by a flower with a normal signal. This is not to suggest, however, that signals can continue to be exaggerated because the physiological or predation costs associated with larger signals would limit the size of a signal a plant is likely to produce.
Studies of foraging behaviour in bees have mostly treated visual signals as stimuli, which the bees use to associate and discriminate rewarding units, and have mostly neglected the possible effects of the signals themselves on foraging (but see Hill et al. 1997; Spaethe et al. 2001; Lynn et al. 2005). Our results show that receiver biases on the part of pollinators might result in foraging behaviour that is in apparent contradiction with the general predictions of optimal foraging theory. Such biases in individual pollinators, amplified in their effect by recruitment in social species, could substantially influence the dynamics of a plant–pollinator system. Plants with an exaggerated signal might be able to draw pollinators in numbers disproportionate to the reward they offer, leading to directional selection for the signal component within a species or population.
Although exaggerated floral signals should regularly appear in a community and have implications for plant competition within it, exaggerations that are well outside the normal range are more likely to be associated with species that are alien to the community. It is therefore possible that alien plant species with the ability to exploit a receiver bias in generalist pollinators like honeybees would have the highest success in invading a native plant–pollinator network. Observations such as that the alien Lythrum salicaria with a larger corolla is more attractive to both native and introduced generalist pollinators than the native Lythrum alatum (Levin 1970) is an indication of such possibilities. While the abundance of generalist pollinators like honeybees has increased in recent times, very little is known about how their behavioural ecology influences native plant–pollinator networks.
Acknowledgments
We sincerely thank M. V. Srinivasan and the referees for their critical input.
Supplementary Material
Learning curves and choice frequencies of individual bees
References
- Arak A, Enquist M. Hidden preferences and the evolution of signals. Phil. Trans. R. Soc. B. 1993;340:207–213. doi:10.1098/rstb.1993.0059 [Google Scholar]
- Basolo A.L. Female preference for male sword length in the green swordtail Xiphophorus helleri (Pisces: Poeciliidae) Anim. Behav. 1990;40:332–338. doi:10.1016/S0003-3472(05)80928-5 [Google Scholar]
- Blarer A, Keasar T, Shmida A. Possible mechanisms for the formation of flower size preferences by foraging bumblebees. Ethology. 2002;108:341–351. doi:10.1046/j.1439-0310.2002.00778.x [Google Scholar]
- Harder L.D, Cruzan M.B. An evaluation of the physiological and evolutionary influences of inflorescence size and flower depth on nectar production. Funct. Ecol. 1990;4:559–572. doi:10.2307/2389323 [Google Scholar]
- Hempel de Ibarra N, Giurfa M, Vorobyev M. Discrimination of coloured patterns by honeybees through chromatic and achromatic cues. J. Comp. Physiol. A. 2002;188:503–512. doi: 10.1007/s00359-002-0322-x. doi:10.1007/s00359-002-0322-x [DOI] [PubMed] [Google Scholar]
- Hill P.S.M, Wells P.H, Wells H. Spontaneous flower constancy and learning in honey bees as a function of colour. Anim. Behav. 1997;54:615–627. doi: 10.1006/anbe.1996.0467. doi:10.1006/anbe.1996.0467 [DOI] [PubMed] [Google Scholar]
- Horridge G.A, Zhang S.-W, Lehrer M. Bees can combine range and visual angle to estimate absolute size. Phil. Trans. R. Soc. B. 1992;337:49–57. doi:10.1098/rstb.1992.0082 [Google Scholar]
- Levin D.A. Assortative pollination in Lythrum. Am. J. Bot. 1970;57:1–5. doi:10.2307/2440373 [Google Scholar]
- Lynn S.K, Cnaani J, Papaj D.R. Peak shift discrimination learning as a mechanism of signal evolution. Evolution. 2005;59:1300–1305. [PubMed] [Google Scholar]
- Makino T.T, Sakai S. Experience changes pollinator responses to floral display size: from size-based to reward-based foraging. Funct. Ecol. 2007;21:854–863. doi:10.1111/j.1365-2435.2007.01293.x [Google Scholar]
- Ryan M.J, Rand A.S. Sexual selection and signal evolution: the ghost of biases past. Phil. Trans. R. Soc. B. 1993;340:187–195. doi:10.1098/rstb.1993.0057 [Google Scholar]
- Spaethe J, Tautz J, Chittka L. Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl Acad. Sci. USA. 2001;98:3898–3903. doi: 10.1073/pnas.071053098. doi:10.1073/pnas.071053098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M.V, Lehrer M. Spatial acuity of honeybee vision, and its spectral properties. J. Comp. Physiol. A. 1988;162:159–172. doi:10.1007/BF00606081 [Google Scholar]
- ten Cate C, Rowe C. Biases in signal evolution: learning makes a difference. Trends Ecol. Evol. 2007;22:380–387. doi: 10.1016/j.tree.2007.03.006. doi:10.1016/j.tree.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Wilkinson G.S, Reillo P.R. Female choice response to artificial selection on an exaggerated male trait in a stalk-eyed fly. Proc. R. Soc. B. 1994;255:1–6. doi:10.1098/rspb.1994.0001 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Learning curves and choice frequencies of individual bees