Abstract
Numerous studies emphasize the potential indirect (genetic) benefits of polyandry in animals with resource-free mating systems. In this paper, we examine the potential for these benefits to fuel sexual selection and polyandry in the hermaphroditic ascidian Pyura stolonifera. Individuals were designated either sire (sperm producers) or dam (egg producers) at random and crossed in a North Carolina II breeding design to produce both paternal and maternal half siblings for our quantitative genetic analysis. We then partitioned the phenotypic variance in fertilization and hatching rates into additive and non-additive variance components. We found significant additive variance attributable to sire and dam effects at fertilization and hatching, suggesting the potential for selection to favour individuals carrying intrinsically ‘good genes’ for these traits. In separate analyses involving monandrous and polyandrous clutches, we found that both traits were elevated under polyandry, but the difference in hatching rates was due entirely to the difference in fertilization rates between treatments. When the hatching rates were standardized to account for variance at fertilization, there was no overall net benefit of polyandry for this trait. Despite this, we found that hatching success declined with increasing embryo densities, and that the slope of this decline was significantly greater in monandrous than polyandrous clutches. Hence, selection on embryo viability may still favour polyandry under restricted environmental conditions. Nevertheless, our results caution against interpreting elevated hatching success as an indirect genetic benefit of polyandry when variance in fertilization is not controlled.
Keywords: compatible genes, multiple mating, sexual selection, North Carolina II, cross-classified
1. Introduction
Evolutionary biologists have long sought adaptive explanations to explain polyandry, when females mate with two or more males within a single reproductive episode (Jennions & Petrie 2000; Simmons 2005). Explaining polyandry in species where there are no obvious direct benefits at mating has proved especially problematic, although in these systems polyandry is thought to result in the procurement of genetic benefits that indirectly increase female fitness via the enhanced genetic quality of their progeny (Jennions & Petrie 2000). Such benefits may arise due to the intrinsic genetic quality of males (additive effects) or beneficial interactions between parental haplotypes (non-additive effects, Neff & Pitcher 2005). Providing unequivocal support for these mechanisms and demonstrating their potential role in the evolution of polyandry has been a key challenge for research on sexual selection (Jennions & Petrie 2000; Simmons 2005).
In this paper, we examine the potential for polyandry and post-mating sexual selection to target genetically compatible and/or intrinsically superior individuals in Pyura stolonifera, a common solitary ascidian found on intertidal rocky shores along the east coast of Australia. Using a cross-classified breeding design (Lynch & Walsh 1998) and split-clutch in vitro fertilization (Evans & Marshall 2005; Marshall & Evans 2005), we partitioned phenotypic sources of variance in fertilization and offspring viability among additive and non-additive effects. Similar designs have recently provided powerful tests of the additive and non-additive benefits of mating with particular males (Wedekind et al. 2001; Pitcher & Neff 2006; Ivy 2007). We combined our quantitative genetic analysis with a polyandry treatment to identify potential fitness benefits of polyandry. Finally, we measured these traits in monandrous and polyandrous clutches across a range of embryo densities to determine whether extrinsic environmental effects have the potential to mediate the benefits of polyandry in this system.
2. Material and methods
Pyura stolonifera is a broadcast spawning marine hermaphrodite that releases eggs and sperm when partly submerged at low tide (Marshall 2002). Although sperm and eggs are released simultaneously, they are not self-fertile (Marshall 2002). Population densities are highly variable in natural populations, which probably explain the high variability in fertilization rates in natural spawnings (ranging from 0 to 90%; Marshall 2002), as fertilization success strongly depends on local sperm concentrations. The density at which embryos develop is also highly variable, with some eggs remaining in a viscous matrix after spawning and others being quickly washed into the water column by incoming waves (Marshall 2002). The larval stage is short (2–4 hours) and larvae show strong settlement preferences for conspecifics (Marshall et al. 2002).
We collected reproductively mature P. stolonifera from the intertidal zone at Bare Island, Botany Bay, Sydney, Australia (151° 23′ E, 39° 99′ S). Adults were maintained in aquaria for no more than 2 days before their gametes were used for in vitro fertilization (IVF). We used the methods described by Marshall and colleagues to collect gametes (Marshall et al. 2000, 2002). Although P. stolonifera is a simultaneous hermaphrodite, we collected gametes of only one type from each individual so that individuals were treated as either ‘male’ or ‘female’. In all IVF trials, sperm were diluted to a constant concentration of 103 sperm per μL−1, which results in a moderate fertilization rates in P. stolonifera (Marshall et al. 2000).
We used a North Carolina II breeding design in conjunction with an experimental polyandry treatment. Briefly, we conducted two separate experimental runs (blocks; Lynch & Walsh 1998). The first consisted of five males and eight females, while the second consisted of six males and five females. For each block, we also included a polyandry treatment, where each female's eggs were fertilized with the combined male ejaculates within each block (for detailed methods, see electronic supplementary material). Two replicate crosses were performed in separate vials for each male–female combination. Fertilization and hatching rates in each cross were estimated across approximately 100 embryos. Overall, for both the factorial crosses and the polyandry treatments, we examined fertilization and hatching rates of 16 600 eggs.
Fertilization was assessed 2 hours after exposing eggs to sperm. Subsamples of approximately 100 eggs were taken from each vial and fixed in formalin (12% v/v in seawater). The remaining developing embryos were maintained at 20°C for 16 h to allow for hatching (Marshall 2002), during which stage they were also fixed in formalin. Fixing eggs and embryos allowed us to estimate fertilization and hatching success accurately without time constraints. Eggs were classed as fertilized if they showed regular cell division. Embryos were classified as successfully hatched when normally developed tadpole larvae emerged. By contrast, unsuccessful hatchings were evident either when they failed to hatch or when hatched offspring exhibited deformities (incomplete development, deformed tails). Although the initial concentration of eggs within each vial was held constant (approx. 1000 eggs per vial), the density of developing embryos varied according to the level of fertilization that occurred within each vial. For data analysis, see electronic supplementary material.
3. Results
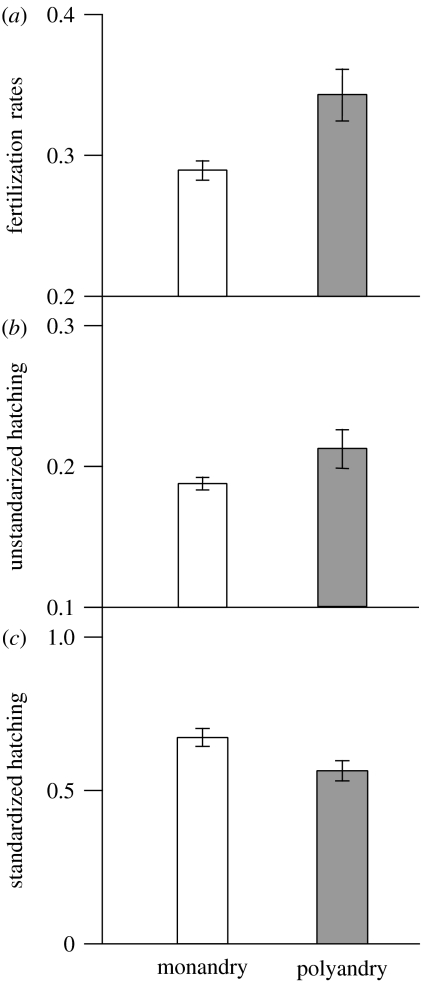
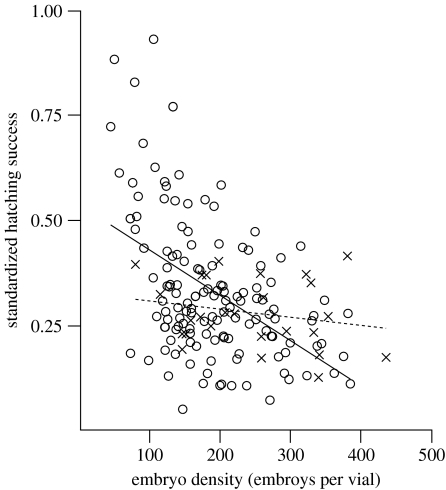
Male and female main effects accounted for significant variance both at fertilization and at hatching (table 1). Although non-significant, there was a trend for an interaction at fertilization (table 1). Fertilization was clearly elevated under polyandry, with eggs accessing the sperm of multiple males achieving 33% higher fertilization success on average than eggs that were exposed to sperm of single males (treatment: F1,151=13.8, p<0.001; block: F1,151=0.2, p=0.675; female (block): F11,151=8.7, p<0.001; figure 1a). Unstandardized hatching success (proportion of initial eggs that resulted in viable offspring) was also significantly higher under polyandry (treatment: F1,150=9.3, p=0.003; block: F1,150=7.1, p=0.009; female (block): F11,151=13.4, p<0.001, figure 1b), but this effect was driven by differences in fertilization rates between monandry and polyandry treatments. When hatching success was standardized to allow for differences in fertilization rates between treatments (proportion of fertilized eggs that subsequently hatched into viable larvae), there was no significant difference in hatching rates between treatments (figure 1c; table 2). Nevertheless, standardized hatching success in both treatments was influenced to different extents by the hatching environment into which embryos emerged: hatching success declined with increasing embryo densities (table 2; figure 2), but the effect of embryo density was less severe in eggs that were fertilized under polyandry than monandry, as revealed by the significant treatment×embryo density interaction (table 2; figure 2). The estimate of the slope for the relationship between embryo density and hatching success under monandry was much steeper than that for the same relationship under polyandry (monandry slope±95% CI: −2.15±−0.66; polyandry slope±95% CI: −0.38±0.72).
Table 1.
Effect of male and female identity on fertilization and standardized hatching rates in P. stolonifera. (The percentage variance explained by each term is calculated from REML output.)
| source | −2 log likelihood | Χ2 | p | % variance |
|---|---|---|---|---|
| fertilization rates | ||||
| male | −319.7 | 3.3 | 0.041 | 11 |
| female | −310.7 | 4.2 | <0.001 | 31 |
| male×female | −320.6 | 13.2 | 0.069 | 17 |
| residual | −323.9 | 41 | ||
| hatching rates | ||||
| male | 53.3 | 4.4 | 0.036 | 10 |
| female | 54.2 | 5.3 | 0.021 | 12 |
| male×female | 49.4 | 0.5 | 0.479 | 6 |
| residual | 48.9 | 72 | ||
Figure 1.
Mean±s.e. of (a) fertilization rates, (b) raw (unstandardized) hatching success and (c) standardized hatching success under monandry and polyandry.
Table 2.
ANCOVA examining the benefits of multiple mating at fertilization for standardized hatching rates across different embryo densities in P. stolonifera. (Note that the model is reduced after testing for non-significant fixed effect by random effect interactions.)
| source | d.f. | mean square | F-ratio | p |
|---|---|---|---|---|
| treatment | 1 | 0.20 | 3.1 | 0.081 |
| embryo density | 1 | 2.83 | 43.0 | <0.001 |
| treatment×embryo density | 1 | 0.34 | 5.1 | 0.025 |
| female (block) | 11 | 0.26 | 3.9 | <0.001 |
| error | 148 | 0.07 |
Figure 2.
The relationship between standardized hatching success (proportion of fertilized eggs that subsequently hatched into viable larvae) and embryo density in monandrous (open circles and solid line) and polyandrous clutches (crosses and dotted line).
4. Discussion
Our analysis of fertilization and hatching revealed significant additive effects attributable to both males and females. Furthermore, our results reveal clear differences in fertilization rates between monandry and polyandry mating treatments, mirroring recent experimental evidence from the sea urchin Heliocidaris erythrogramma (Evans & Marshall 2005) and the serpulid polychaete Galeolaria caespitosa (Marshall & Evans 2005). Unlike these previous studies, however, male–female interactions did not significantly mediate fertilization success.
Our use of an externally fertilizing broadcast spawner to investigate the benefits of polyandry at fertilization and hatching allowed us to compare unstandardized (raw) and standardized (corrected for fertilization) hatching rates between mating treatments. The discrepancy between the results from both analyses illustrates a clear need to account for variance in fertilization when estimating hatching rates. Recent advances in applying NCII designs to vertebrate models (fishes), where it is possible to estimate fertilization directly and distinguish early and late embryo mortality (Wedekind et al. 2001; Pitcher & Neff 2006), offer excellent promise for extending our findings to other systems, where it is possible to manipulate polyandry experimentally. To date, however, the majority of studies reporting differences in hatching rates between monandrous and polyandrous clutches focus on egg-laying species and do not account for variance in initial fertilization and pre-hatching survival when comparing hatching rates. In other cases, however, the presumed indirect genetic benefits of polyandry might actually reflect direct fertilization benefits (as in our study). Our work therefore cautions against interpreting elevated hatching success as an indirect genetic benefit of polyandry when variance in fertilization is not controlled. It also bolsters ongoing arguments stating that hatching success (or paternity estimates derived from hatchlings) may be an unreliable measure of embryo viability (Gilchrist & Partridge 1997; Olsson et al. 1999; García-González & Simmons 2007).
Finally, our finding that standardized hatching success declined with increasing embryo densities, and that the slope of this decline was significantly greater in monandrous than polyandrous clutches, suggests that selection on embryo viability may still favour polyandry under restricted environmental conditions (figure 2). These context-dependent benefits of polyandry are likely to be biologically relevant in P. stolonifera, where densities of developing embryos vary dramatically in natural populations (Marshall 2002). We can only speculate on the mechanisms underlying these interacting effects of embryo density and mating treatment, although promising avenues for future research include the possibility that under increasingly stressful conditions (competition), higher levels of genetic diversity (as in the polyandry treatment) are favoured (Williams & Mitton 1973).
Acknowledgments
We thank Bronwyn Cumbo for excellent assistance in the laboratory and Peter Steinberg for graciously providing laboratory space for this work. Also, thanks to Michael Jennions and two anonymous reviewers for their comments that greatly improved the manuscript. This work was supported by a vice chancellor's research fellowship and Australian Research Council grants to D.J.M. and J.P.E.
Supplementary Material
Supplementary methods
References
- Evans J.P, Marshall D.J. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution. 2005;59:106–112. [PubMed] [Google Scholar]
- García-González F, Simmons L.W. Paternal indirect effects on offspring viability and the benefits of polyandry. Curr. Biol. 2007;17:32–36. doi: 10.1016/j.cub.2006.10.054. doi:10.1016/j.cub.2006.10.054 [DOI] [PubMed] [Google Scholar]
- Gilchrist A.S, Partridge L. Heritability of pre-adult viability differences can explain apparent heritability of sperm displacement ability in Drosophila melanogaster. Proc. R. Soc. B. 1997;264:1271–1275. doi: 10.1098/rspb.1997.0175. doi:10.1098/rspb.1997.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy T.M. Good genes, genetic compatibility and the evolution of polyandry: use of the diallel cross to address competing hypotheses. J. Evol. Biol. 2007;20:479–487. doi: 10.1111/j.1420-9101.2006.01269.x. doi:10.1111/j.1420-9101.2006.01269.x [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Sinauer Associates, Inc; Sunderland, MA: 1998. Genetics and analysis of quantitative traits. [Google Scholar]
- Marshall D.J. In situ measures of spawning synchrony and fertilization in an intertidal, free-spawning invertebrate. Mar. Ecol. Prog. Ser. 2002;236:113–119. doi:10.3354/meps236113 [Google Scholar]
- Marshall D.J, Evans J.P. The benefits of polyandry in the free-spawning polychaete Galeolaria caespitosa. J. Evol. Biol. 2005;18:735–741. doi: 10.1111/j.1420-9101.2004.00873.x. doi:10.1111/j.1420-9101.2004.00873.x [DOI] [PubMed] [Google Scholar]
- Marshall D.J, Styan C.A, Keough M.J. Intraspecific co-variation between egg and body size affects fertilisation kinetics in free-spawning marine invertebrates. Mar. Ecol. Prog. Ser. 2000;195:305–309. doi:10.3354/meps195305 [Google Scholar]
- Marshall D.J, Styan C.A, Keough M.J. Sperm environment affects offspring quality in broadcast spawning marine invertebrates. Ecol. Lett. 2002;5:173–176. doi:10.1046/j.1461-0248.2002.00257.x [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Olsson M, Pagel M, Shine R, Madsen T. Sperm choice and sperm competition: suggestions for field and laboratory studies. Oikos. 1999;84:172–175. doi:10.2307/3546880 [Google Scholar]
- Pitcher T.E, Neff B.D. MHC class IIB alleles contribute to both additive and non-additive genetic effects on survival in Chinook salmon. Mol. Ecol. 2006;15:2357–2365. doi: 10.1111/j.1365-294X.2006.02942.x. doi:10.1111/j.1365-294X.2006.02942.x [DOI] [PubMed] [Google Scholar]
- Simmons L.W. The evolution of polyandry: sperm competition, sperm selection and offspring viability. Annu. Rev. Ecol. Evol. Syst. 2005;36:125–146. doi:10.1146/annurev.ecolsys.36.102403.112501 [Google Scholar]
- Wedekind C, Muller R, Spicher H. Potential genetic benefits of mate selection in whitefish. J. Evol. Biol. 2001;14:980–986. doi:10.1046/j.1420-9101.2001.00349.x [Google Scholar]
- Williams G.C, Mitton J.B. Why reproduce sexually? J. Theor. Biol. 1973;39:545–554. doi: 10.1016/0022-5193(73)90067-2. doi:10.1016/0022-5193(73)90067-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods