Summary
Background
Therapeutic hypothermia (TH) represents an important method to attenuate post-resuscitation injury after cardiac arrest. Laboratory investigations have suggested that induction of hypothermia before return of spontaneous circulation (ROSC) may confer the greatest benefit. We hypothesized that a short delay in resuscitation to induce hypothermia before ROSC, even at the expense of more prolonged ischemia, may yield both physiological and survival advantages.
Methods
Cardiac arrest was induced in C57BL/6 mice using intravenous potassium chloride; resuscitation was attempted with CPR and fluid administration. Animals were randomized into three groups (n=15 each): a normothermic control group, in which 8 min of arrest at 37°C was followed by resuscitation; an early intra-arrest hypothermia group, in which 6.5 min of 37°C arrest were followed by 90 sec of cooling, with resuscitation attempted at 30°C (8 min total ischemia); and a delayed intra-arrest hypothermia group, with 90 sec cooling begun after 8 min of 37°C ischemia, so that animals underwent resuscitation at 9.5 min.
Results
Animals treated with TH demonstrated improved hemodynamic variables and survival compared to normothermic controls. This was the case even when comparing the delayed intra-arrest hypothermia group with prolonged ischemia time against normothermic controls with shorter ischemia time (7 day survival, 4/15 vs 0/15, p<0.001).
Conclusions
Short resuscitation delays to allow establishment of hypothermia before ROSC appear beneficial to both cardiac function and survival. This finding supports work suggesting that post-resuscitation injury processes begin immediately after ROSC, and that intra-arrest cooling may serve as a useful therapeutic approach to improve survival.
Keywords: cardiopulmonary resuscitation, heart arrest, induced hypothermia, reperfusion injury
Introduction
Prompt delivery of CPR and early defibrillation to achieve return of spontaneous circulation (ROSC) remain the highest priorities of immediate care for the cardiac arrest victim as elaborated in the 2005 consensus resuscitation guidelines.1 However, survival from cardiac arrest remains low despite widespread efforts at improved defibrillation and CPR quality, both fundamental strategies to generate rapid reperfusion.2,3
Therapeutic hypothermia (TH) has been shown to improve both survival and neurological outcomes after cardiac arrest in several clinical trials, and has been endorsed as an evidence-based treatment modality by the International Liaison Committee on Resuscitation.4–7 Currently TH is applied in the post-resuscitation time period, to minimize reperfusion injury following ischemia and improve neurological outcome after ROSC is achieved.8 Earlier animal work by our group and others has suggested that intra-arrest hypothermia may provide additional survival benefit compared to TH after resuscitation.9,10 Cellular ischemia-reperfusion experiments have observed deleterious oxidant generation as well as apoptotic activation immediately after reperfusion, and intra-ischemia cooling blunts these damaging processes.11–13 It is unknown to what degree initial cooling should be given priority over reperfusion and ROSC, if at all. This is particularly controversial if the induction of TH requires a delay in circulatory resuscitation and restoration of perfusion. We sought to test the hypothesis that intra-arrest TH, and therefore “cooled reperfusion”, would lead to improved cardiac function and survival in an animal model of cardiac arrest even if resuscitation efforts, and therefore ROSC, were delayed to facilitate cooling before CPR.
Methods
Animal Preparation
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Chicago (IACUC #71041). Animal husbandry was supervised by the Animal Research Center veterinary staff and included routine care for animals surviving cardiac arrest.
As described in our earlier work, we used adult female C57BL/6 mice (25 – 33 g; Taconic Farms, Germantown, NY). Animals were allowed free access to food and water prior to study. Animals were anesthetized with 80 µg/g of ketamine (Phoenix Scientific, Inc. St. Joseph, MO) and 12 µg/g xylazine (Ben Venue Laboratories, Bedford, Ohio) via intraperitoneal injection. During surgical preparation, body temperature was maintained at 37 °C via a heating lamp and monitored via a rectal thermocouple probe (Omega Engineering Inc., Stamford, CT) coupled to a digital recording thermometer (Fluke II-2, Fluke Corp., Everett, WA). The trachea was intubated orally with a 20-gauge catheter (Angiocath, Becton Dickinson, Sandy, UT). After the tracheal tube position was verified visually and secured, mechanical ventilation was performed using a small-volume ventilator (Flexivent EC-VF-2, Scireq Scientific Respiratory Equipment, Montreal, Canada). Ventilator settings included a tidal volume of 12.5 µl/g, a respiratory rate of 110 breaths per min, a FiO2 of 1.0, and a positive end-expiratory pressure of 2 cm H20. Left ventricular pressure and volume were measured with a Millar P-V catheter (SPR-839, P/N 840-8111, Millar Instruments, Houston, TX) inserted via the right carotid artery into the left ventricle. A saline-filled microcatheter (EZ-1101, BioTime Inc., Berkeley, CA) was inserted into the left jugular vein for fluid administration. End-tidal CO2 (ETCO2) was measured inline with the tracheal tube (Micro-Capnometer, Columbus Instruments, Columbus, OH). Needle-probe ECG monitoring data and ETCO2 were recorded using a PC-based data acquisition system (DI-720, DI-205, Windaq, DATAQ Instruments, Inc., Akron, OH).
Invasive Monitoring of Cardiac Function
The Millar Instruments Pressure-Volume (P-V) conductance system (MPCU-200, P/N 880-0118) was used in conjunction with the ultra-miniature Millar P-V catheter to generate simultaneous pressure-volume data, set at a signal frequency of 20 kHz and an output current of 20 µA. The MPCU-200's integrated pressure channel output was fixed at 1V/100mmHg. The pressure and volume signals were acquired using a data acquisition system (PowerLab 4/SP, ADInstruments, Colorado Springs, CO) at a sampling rate of 1 kHz. The conductance/volume data were acquired as a voltage signal and converted into Relative Volume Units using the built-in electronic calibration settings on the MPCU-200, and then later converted into true volume units (µL) using a linear relationship derived from a calibration system described by the manufacturer. Parametric indices of left ventricular function including: isovolumic contractility (dP/dt), isovolumic relaxation time constant (tau) and cardiac output were derived from P-V catheter data using Millar PVAN 3.0 analytic software.
Experimental Groups
Animals were randomized after successful surgical preparation into three groups using the blinded drawing of group assignment slips out of a container, n=15 per group (see Figure 1 for schematic of experimental groups). The groups were comprised of: (1) a normothermic control group, in which animals underwent 8 min of untreated arrest, after which time resuscitation was attempted while body temperature was maintained at 37°C, (2) an early intra-arrest hypothermia group (EIH), in which animals underwent 6.5 min of untreated cardiac arrest and were then cooled without reperfusion to a target temperature of 30°C over an additional 1.5 min (8 min total arrest time), after which time resuscitation was attempted, cooling was maintained for one hour, and re-warming to 37°C took place at one hour post-ROSC. These two animal groups were compared with (3) a delayed intra-arrest hypothermia (DIH) group, in which animals underwent 8 min of untreated cardiac arrest and received cooling at this point, resulting in 9.5 min of total arrest time before resuscitation began. Again, cooling was maintained for one hour, and re-warming to 37°C then initiated. One hour cooling duration was chosen for convenience based on our earlier experimental work that demonstrated this duration to be sufficient in providing measurable clinical benefit.10
Figure 1.
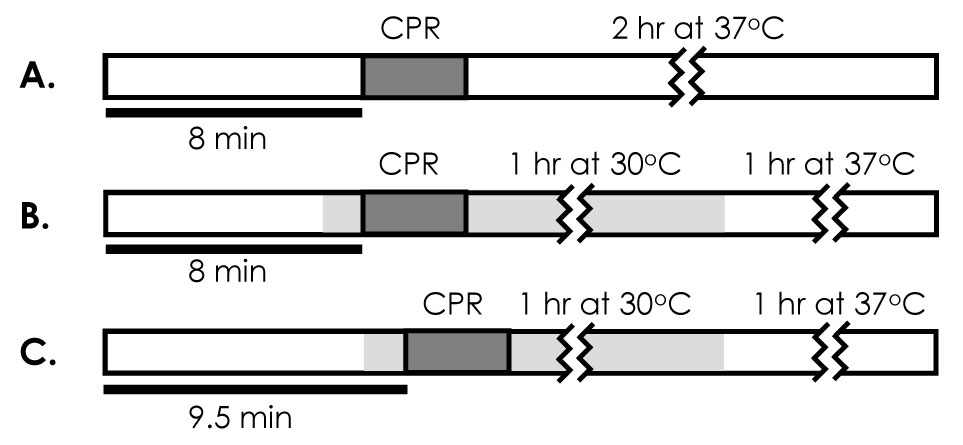
Schematic of experimental protocol. Thick horizontal lines show duration of arrest for each group, dark grey box represent CPR interval, and light grey area represents interval of therapeutic hypothermia. A, normothermic control group with 8 min arrest duration, approximately 3–4 min of CPR and 2 hrs of monitored recovery at 37°C. B, early intra-arrest (EIH) cooling group with 8 min arrest duration and 3–4 min CPR interval. In addition, cooling is initiated at 6.5 min of arrest such that resuscitation occurs at 30°C. After one hour, animals are rewarmed and monitored for 1 hour at 37°C. C, delayed intra-arrest (DIH) cooling group, with 9.5 min arrest duration and 3–4 min CPR interval. Cooling is initiated at 8 min of arrest such that resuscitation occurs at 30°C. After one hr, animals are rewarmed and monitored for an additional hr at 37°C.
Following surgical preparation, animals were connected to the ventilator and monitored until 50 min had elapsed from time of anesthesia induction. At this point, the P-V catheter was withdrawn carefully into the descending aorta to measure baseline arterial blood pressures. Animals were excluded for mean arterial pressures less than 80 mmHg, ETCO2 less than 35 mmHg or for overt surgical complications such as failed cannulation or excessive bleeding. Cardiac arrest was then induced by giving 0.08 mg/g potassium chloride solution (Sigma, St. Louis, MO) via the jugular catheter. In earlier work, we found this dose sufficient to induce a state of asystolic arrest that persisted unless resuscitation is attempted. After either 8 min (control and EIH groups) or 9.5 min (DIH group) of untreated asystolic arrest, resuscitation was attempted by giving chest compressions and ventilations for up to 5 min. Chest compressions were delivered manually at a rate of approximately 400 beats/ min for all animals by the same investigator. Consistency of chest compressions was maintained by adjusting to provide a uniform rate as visualized on the ECG monitor and a target aortic diastolic pressure of greater than 20 mm Hg. Hypothermia was induced using direct contact with a “cooling blanket” fashioned from an ice-water filled surgical glove; this method has previously been validated to provide cooling to 30°C within 90 seconds. Target temperature was maintained via careful positioning of the cooling blanket and heat lamp to maintain a temperature of 30±0.5 °C during cooling and 37.0±0.5 °C in normothermia. ROSC was defined as the return of sinus rhythm with a mean arterial pressure of greater than 40 mm Hg lasting for at least 5 min. Resuscitation efforts were terminated after 5 min or upon hemodynamic evidence of initial ROSC.
For animals achieving initial ROSC, the pressure-volume sensing catheter was again advanced into the left ventricle and measurements were recorded every 10 min. Resuscitated animals were monitored and mechanically ventilated for two hours. All catheters were then removed, wounds were closed surgically, and animals were extubated. During the two hour observation period, all animals received approximately 0.5 ml 0.9% saline intravenously. Following extubation, animals were observed continuously under a heating lamp with temperature monitoring and received an intraperitoneal injection of 0.5 ml 0.9% saline during the first hour after extubation. All survivors were assessed neurologically each day as described below and were then sacrificed after seven days.
Neurologic Scoring
The mice underwent neurological assessment at six hours and then on post-arrest days 1, 2 and 3. Neurological function was evaluated following a modified scoring system for rodents published previously 14 15 and used by our group in earlier work.10. Two investigators performed the neurological evaluation and consensus scores were established.
Statistical Analysis
Mouse characteristics including hemodynamic data are reported as mean ± SD except where otherwise noted. Comparisons between groups were made using one-way analysis of variance (ANOVA). Comparisons of non-normally-distributed data were made using Kruskal-Wallis ANOVA on ranks with post-hoc Dunn’s testing. The Chi-square test was used to compare ROSC rates between groups. Survival outcomes were compared via log-rank analysis. Statistical significance was defined as p< 0.05. Statistical computations were performed using STATA 9.0 (StataCorp, College Station, TX).
Experimental Results
A total of 49 mice underwent surgical preparation to yield 45 animals randomized for the three experimental groups. Baseline characteristics (Table 1) including animal weight, temperature, heart rate and left ventricular (LV) pressure before arrest were statistically indistinguishable between groups. During the arrest period, cooling rates did not differ significantly between the two hypothermia groups (Table 1). We observed that animals cooled rapidly to the target temperature within 90 seconds (consistent with our earlier work), allowing for animals to reach 30 °C before resuscitation with chest compression was attempted. Measures of CPR quality such as chest compression rate and duration, as well as average diastolic blood pressure (DBP) over the first two min of resuscitation were compared and found to be equivalent between groups. The percentage of animals achieving initial ROSC was also indistinguishable among the groups.
Table 1.
Group characteristics (mean ± SD) during baseline, arrest and resuscitation periods; and at 120 min following initial resuscitation, n=15 per group. Significant differences between control and hypothermia groups (*) and between the hypothermia groups (EIH and DIH) are denoted (¥) for p-value < 0.05. Chest compression (CC) rate and duration; diastolic blood pressure (DBP) and ETCO2 were averaged over the first 2 min of resuscitation. Abbreviations: maximum left ventricular pressure (LVPmax), end-tidal CO2 (ETCO2), return of spontaneous circulation (ROSC), not applicable (NA).
| Control | EIH | DIH | ||
|---|---|---|---|---|
| Baseline | Weight | 29.5±2.0 | 29.2±1.4 | 29.0±1.5 |
| Temperature, °C | 36.4±0.3 | 36.4±0.4 | 36.5±0.4 | |
| Heart rate, bpm | 280.8±65.7 | 251.8±43.0 | 256.8±53.5 | |
| LVPmax, mmHg | 94.4±8.5 | 92.3±7.2 | 92.0±7.4 | |
| ETCO2, mmHg | 36.1±3.8 | 36.6±3.4 | 37.1±2.5 | |
| Arres | Cooling rate | NA | 5.1±1.9 | 5.3±1.8 |
| Warming rate | NA | 0.23±0.4 | 0.26±0.6 | |
| Resuscitation | ROSC, n (%) | 12 (80) | 14 (93) | 14 (93) |
| CC rate, bpm | 394±63.4 | 382±35.4 | 377±40.6 | |
| CC duration, s | 190.7±68.9 | 158.3±58.8 | 162.7±48.9 | |
| DBP, mmHg | 15.3±4.2 | 16.2±7.0 | 16.2±7.6 | |
| ETCO2, mmHg | 24.5±5.8 | 20.5±5.6* | 21.1±6.6* | |
| 120 min | Heart rate, bpm | 476.6±48.0 | 383.2±100.6 | 373.2±130.8 |
| LVPmax, mmHg | 60.1±10.2 | 80.2±8.6*¥ | 70.6±10.3* | |
| ETCO2, mmHg | 34.8±3.2 | 35.3±2.0 | 35.2±3.3 | |
| Survival, n (%) | 8 (53) | 14 (93) | 14 (93) | |
Figure 2 displays representative P-V loops for animals at baseline and at 30, 60 and 120 min following resuscitation. In both experimental groups, intra-arrest hypothermia resulted in qualitatively improved cardiovascular parameters including LVPmax, cardiac output, left ventricular dP/dtmax as illustrated in Figure 3. These differences were statistically significant at the end of the 120 min post-resuscitation period. Note that while cardiac output in the DIH group was higher relative to control this trend did not reach statistical significance. Earlier cooling was associated with significantly improved hemodynamics as LVPmax, dP/dtmax and cardiac output were significantly improved in the EIH as compared to the DIH group. Interestingly, the time constant of isovolumic relaxation (tau), was transiently higher in the hypothermia groups during the post-resuscitation period of hypothermia (Figure 3D). This relationship reversed at 120 min, as and was found to be significantly elevated in the control group relative to both of the hypothermia groups. Mice in both intra-arrest hypothermia-treated groups exhibited transiently lower ETCO2 early in the post-resuscitation period, consistent with lowered metabolic rate induced by cooling (data not shown).
Figure 2.
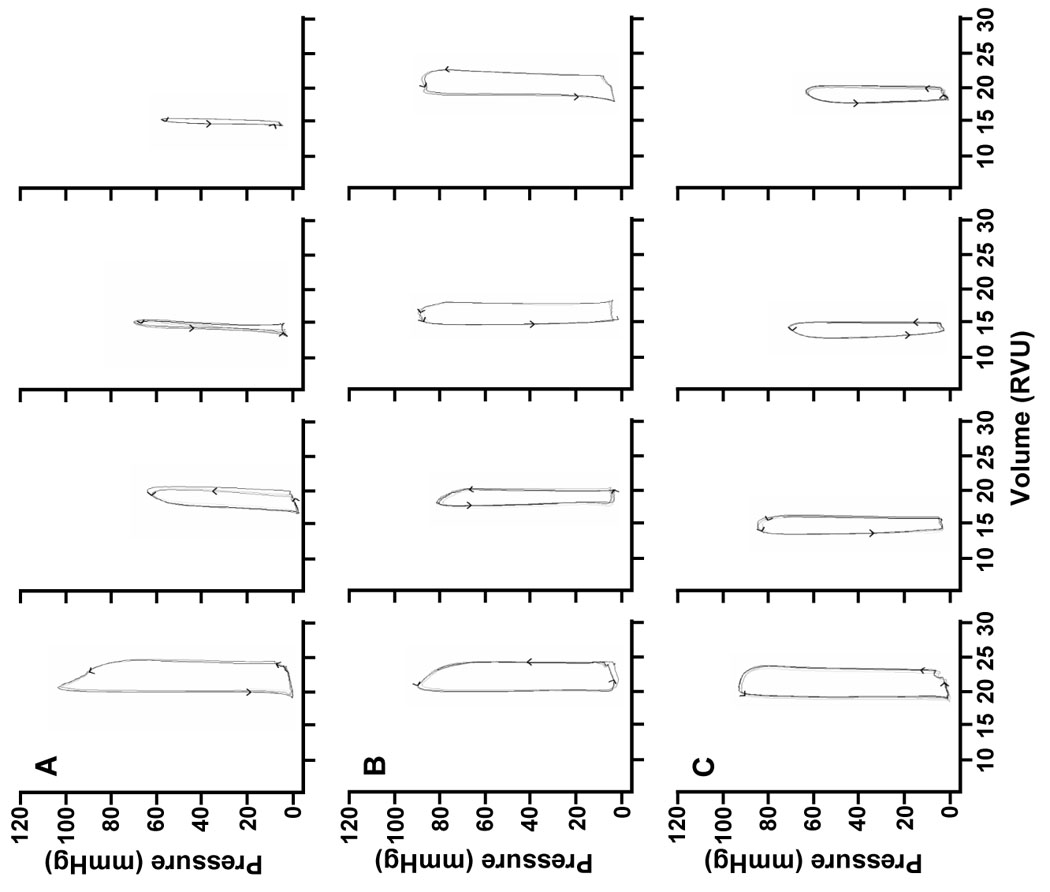
Representative left ventricular pressure-volume (P-V) loops measured at baseline, 30, 60 and 120 min post-ROSC from (A) control, (B) early intra-arrest hypothermia (EIH) and (C) delayed intra-arrest hypothermia (DIH) experimental groups.
Figure 3.

Cardiac physiological measurements, taken at baseline 10 min before cardiac arrest, and then at 10 min intervals during protocol including (A) peak left ventricular pressures (LVPmax); (B) dP/dtmax, a measure of contractility; (C) cardiac output, and (D) the isovolumic relaxation time constant, tau. Statistically significant differences between both hypothermia groups and controls (*) and between EIH and DIH groups (¥) are denoted for p < 0.05.
There was a significant survival benefit demonstrated in both intra-arrest hypothermia groups at 6 hours which persisted to 7 days (Figure 4). Furthermore, at 7 days, early cooling (EIH) improved survival relative to delayed (DIH) cooling. Consistent with our earlier work, cooled animals from both hypothermia groups demonstrated improved neurological function compared to normothermic animals when assessed initially (at 6 hours after arrest), but this difference in neurological performance diminished with time in survivors (Figure 5).
Figure 4.

Survival after cardiac arrest. Kaplan-Meier survival plot of normothermic control group and the two experimental groups are shown. Statistically significant differences between both hypothermia groups and controls (*) and between EIH and DIH groups (¥) are denoted at 6, 24 and 72 hrs and at 7 days.
Figure 5.

Neurological function scores after resuscitation. Each circle represents an individual animal scored at a specific time after resuscitation. A, 6 hrs after ROSC. B, 24 hrs after ROSC. C, 72 hrs after ROSC. The neurological scoring system has been detailed previously.10 Open circles represent early intra-arrest hypothermia (EIH) animals; grey circles represent delayed intra-arrest hypothermia (DIH) animals; black circles represent normothermic controls.
Discussion
In the present study we used a mouse model of cardiac arrest to demonstrate that hemodynamic, survival and neurological metrics were significantly improved with intra-arrest cooling even if resuscitation was delayed for 90 seconds to first induce hypothermia. Specifically, animals undergoing normothermic resuscitation at 8 min fared worse than animals with delayed resuscitation and cooled reperfusion at 9.5 min (with identical 8 min of normothermic arrest time in both groups). This is consistent with the notion of lethal reperfusion injury as we observe that a somewhat longer period of ischemia is paradoxically less deleterious than the reperfusion injury that occurs with earlier resuscitation in the normothermic state.
It is interesting to note that while all the groups had nearly identical ROSC rates, myocardial contractility was significantly decreased in animals that did not receive hypothermia treatment. This cardiovascular reperfusion injury resulted in considerably reduced cardiac output with concomitant systolic and diastolic myocardial dysfunction during the post-arrest period. These findings are concordant with earlier animal investigations, and such impaired myocardial function likely serves as a contributor to post-arrest injury and mortality observed clinically.16–18 It is also interesting to note that one hour of cooling after ROSC was sufficient to generate a measurable clinical benefit, as shown in our prior work.10 Whether shorter duration of cooling is sufficient in a clinical setting is currently unknown.
These findings are consistent with our prior work on cardiomyocyte ischemia-reperfusion in vitro, in which we have found that a longer period of ischemia that includes intra-ischemia cooling prior to reperfusion confers improved cell viability and attenuates a number of intracellular injury pathway mechanisms, including the apoptotic enzyme caspase-3, when compared to faster reperfusion but without cooling.13 Other animal work has demonstrated that the earlier hypothermia therapy is instituted after an ischemic insult, the more likely survival and surrogate outcome measures will be improved. 10, 19–21 Finally, human investigations have suggested that the induction of hypothermia is a time-sensitive intervention and that injury pathways may be activated very shortly after ROSC.5,6 Taken together, it appears that attempts at induction of TH before reperfusion may be an important concept with which to challenge the current guidelines for resuscitation and post-resuscitation care. While most studies of TH for cardiac arrest have focused on its associated neuroprotection, cooling induced early with CPR could have both cardioprotective and neuroprotective properties.10 This notion is consistent with work by Nozari et al who studied the therapeutic effect of hypothermia induced after 20 min of warm global cardiac ischemia in a canine model of ventricular fibrillation. In this model of end-ischemia cooling, both heart and brain demonstrated hypothermia protection.22
There are a number of important limitations in this work especially regarding the translation of these data to the clinical setting. The time required for TH induction in the mouse was 90 seconds. This is not currently feasible in humans and it is likely that much longer resuscitation delays in the clinical setting might counteract the benefit of cooling before ROSC. Further research into the time kinetics of cooling will be required. The speed of cooling is a technological hurdle that is likely to diminish in the future, as newer cooling devices and methods become available. For example, the immediate use of ice-cold intravenous saline to induce TH has been shown to be feasible and can achieve target temperatures (32–34°C) within 60 min.23,24 It is possible that the use of newer devices and adjunctive use of cold saline might allow for partial cooling to be achieved in an even shorter time frame. In addition, while a number of groups have demonstrated the value of the mouse model of cardiac arrest to evaluate post-resuscitation injury,25–27 further work in larger animal models will be required. Of note, some have reported that rapid cooling of the heart during CPR is at least possible in larger animal models. Zviman et al reported that use of an intravenous cooling device in swine (25–37kg) could cool the heart to 34 °C within 4 min of CPR.28
Conclusion
Delayed intra-arrest cooling may provide significant hemodynamic, neurological and survival benefits compared with earlier reperfusion without cooling, thus the current paradigm that gives priority to prompt ROSC first may need further careful evaluation. Evaluation of the mechanistic effects of this intra-ischemic cooling will allow for a better understanding of how it may be applied in the clinical setting.
Supplementary Material
Acknowledgements
We would like to thank Raina Merchant, MD for advice and critique of the current work. We also thank Michael Retzer, Lynne Harnish, and Ameena Al-Amin for their administrative assistance. This work was supported by a grant from the National Institutes of Health (R01-HL71734-01). Dr. Abella is also supported by a Career Development Award from the NIH (1 K23 HL 83082-01).
Footnotes
Authorship Contributions Conception of study: BS Abella, LB Becker, TL Vanden Hoek, D Zhao, Experimental work: BS Abella, JP Alvarado, H Wang, D Zhao, Data analysis: BS Abella, DG Beiser, D Zhao, Statistical treatment: DG Beiser, D Zhao, Manuscript preparation: BS Abella, LB Becker, DG Beiser, K Hamann, TL Vanden Hoek, D Zhao
Conflict of Interest Dr. Abella has received speaking honoraria from Philips Medical Systems, Zoll Corporation and Alsius corporation, and research funding from Philips Medical Systems and Cardiac Science Corporation. Dr. Becker has received research funding from Alsius Corporation, Philips Medical Systems, and Cardiac Science Corporation. Dr. Vanden Hoek has received grant support from Philips Medical Systems and Medivance Incorporated. Drs. Becker and Vanden Hoek have equity interest in Cold Core Therapeutics, as well as intellectual property in the area of coolant technology for clinical use. The remaining authors do not declare any relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.2005 International Consensus on Cardiopulmonary Resuscitation (CPR) and Emergency Cardiovascular Care (ECC) Science With Treatment Recommendations. Circulation. 2005;112 suppl III:III-1–III-136. [Google Scholar]
- 2.Eckstein M, Stratton SJ, Chan LS. Cardiac Arrest Resuscitation Evaluation in Los Angeles: CARE-LA. Ann Emerg Med. 2005;45:504–509. doi: 10.1016/j.annemergmed.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Dunne RB, Compton S, Zalenski RJ, Swor R, Welch R, Bock BF. Outcomes from out-of-hospital cardiac arrest in Detroit. Resuscitation. 2007;72:59–65. doi: 10.1016/j.resuscitation.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation. 2001;51:275–281. doi: 10.1016/s0300-9572(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 5.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 6.Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 7.Nolan JP, Morley PT, Vanden Hoek TL, Hickey RW, Kloeck WG, Billi J, Bottiger BW, Morley PT, Nolan JP, Okada K, Reyes C, Shuster M, Steen PA, Weil MH, Wenzel V, Hickey RW, Carli P, Vanden Hoek TL, Atkins D. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation. Circulation. 2003;108:118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 8.Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Mullner M. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta-analysis. Crit Care Med. 2005;33:414–418. doi: 10.1097/01.ccm.0000153410.87750.53. [DOI] [PubMed] [Google Scholar]
- 9.Leonov Y, Sterz F, Safar P, Radovsky A, Oku K, Tisherman S, Stezoski SW. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10:57–70. doi: 10.1038/jcbfm.1990.8. [DOI] [PubMed] [Google Scholar]
- 10.Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- 11.Vanden Hoek TL, Shao Z, Li C, Schumacker PT, Becker LB. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J Mol Cell Cardiol. 1997;29:2441–2450. doi: 10.1006/jmcc.1997.0481. [DOI] [PubMed] [Google Scholar]
- 12.Vanden Hoek TL, Qin Y, Wojcik K, Li CQ, Shao ZH, Anderson T, Becker LB, Hamann KJ. Reperfusion, not simulated ischemia, initiates intrinsic apoptosis injury in chick cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;284:H141–H150. doi: 10.1152/ajpheart.00132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao ZH, Chang WT, Chan KC, Wojcik KR, Hsu CW, Li CQ, Li J, Anderson T, Qin Y, Becker LB, Hamann KJ, Vanden Hoek TL. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol Heart Circ Physiol. 2007;292:H1995–H2003. doi: 10.1152/ajpheart.01312.2005. [DOI] [PubMed] [Google Scholar]
- 14.Hendrickx HH, Rao GR, Safar P, Gisvold SE. Asphyxia, cardiac arrest and resuscitation in rats. I. Short term recovery. Resuscitation. 1984;12:97–116. doi: 10.1016/0300-9572(84)90062-5. [DOI] [PubMed] [Google Scholar]
- 15.Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- 16.Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: a 3-phase time-sensitive model. JAMA. 2002;288:3035–3038. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 17.Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 18.Rincon F, Mayer SA. Therapeutic hypothermia for brain injury after cardiac arrest. Semin Neurol. 2006;26:387–395. doi: 10.1055/s-2006-948319. [DOI] [PubMed] [Google Scholar]
- 19.Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Markarian GZ, Lee JH, Stein DJ, Hong SC. Mild hypothermia: therapeutic window after experimental cerebral ischemia. Neurosurgery. 1996;38:542–550. doi: 10.1097/00006123-199603000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Markgraf CG, Clifton GL, Moody MR. Treatment window for hypothermia in brain injury. J Neurosurg. 2001;95:979–983. doi: 10.3171/jns.2001.95.6.0979. [DOI] [PubMed] [Google Scholar]
- 22.Nozari A, Safar P, Stezoski SW, Wu X, Henchir J, Radovsky A, Hanson K, Klein E, Kochanek PM, Tisherman SA. Mild hypothermia during prolonged cardiopulmonary cerebral resuscitation increases conscious survival in dogs. Crit Care Med. 2004;32:2110–2116. doi: 10.1097/01.ccm.0000142700.19377.ae. [DOI] [PubMed] [Google Scholar]
- 23.Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 24.Kliegel A, Janata A, Wandaller C, Uray T, Spiel A, Losert H, Kliegel M, Holzer M, Haugk M, Sterz F, Laggner AN. Cold infusions alone are effective for induction of therapeutic hypothermia but do not keep patients cool after cardiac arrest. Resuscitation. 2007;73:46–53. doi: 10.1016/j.resuscitation.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 25.Bottiger BW, Teschendorf P, Krumnikl JJ, Vogel P, Galmbacher R, Schmitz B, Motsch J, Martin E, Gass P. Global cerebral ischemia due to cardiocirculatory arrest in mice causes neuronal degeneration and early induction of transcription factor genes in the hippocampus. Mol Brain Res. 1999;65:135–142. doi: 10.1016/s0169-328x(98)00298-8. [DOI] [PubMed] [Google Scholar]
- 26.Song L, Weil MH, Tang W, Sun S, Pellis T. Cardiopulmonary resuscitation in the mouse. J Appl Physiol. 2002;93:1222–1226. doi: 10.1152/japplphysiol.01079.2001. [DOI] [PubMed] [Google Scholar]
- 27.Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol. 2003;285:F87–F94. doi: 10.1152/ajprenal.00026.2003. [DOI] [PubMed] [Google Scholar]
- 28.Zviman MM, Roguin A, Jacobs A, Rent K, Lardo A, Halperin HR. A new method for inducing hypothermia during cardiac arrest. Crit Care Med. 2004;32:S369–S373. doi: 10.1097/01.ccm.0000139461.46564.51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


