Abstract
M/KCNQ currents play a critical role in the determination of neuronal excitability. Many neurotransmitters and peptides modulate M/KCNQ current and neuronal excitability through their G protein–coupled receptors. Nerve growth factor (NGF) activates its receptor, a member of receptor tyrosine kinase (RTK) superfamily, and crucially modulates neuronal cell survival, proliferation, and differentiation. In this study, we studied the effect of NGF on the neuronal (rat superior cervical ganglion, SCG) M/KCNQ currents and excitability. As reported before, subpopulation SCG neurons with distinct firing properties could be classified into tonic, phasic-1, and phasic-2 neurons. NGF inhibited M/KCNQ currents by similar proportion in all three classes of SCG neurons but increased the excitability only significantly in tonic SCG neurons. The effect of NGF on excitability correlated with a smaller M-current density in tonic neurons. The present study indicates that NGF is an M/KCNQ channel modulator and the characteristic modulation of the neuronal excitability by NGF may have important physiological implications.
INTRODUCTION
M/KCNQ potassium currents were first described in sympathetic neurons (Brown and Adams, 1980). Subsequently, M/KCNQ currents have been recorded in mammalian brain neurons, including pyramidal cells of the hippocampus (Halliwell and Adams, 1982). M/KCNQ currents play a crucial role in the stabilization of membrane potential and the modulation of neuronal excitability (Marrion, 1997; Jentsch, 2000). It is now established that KCNQ2/Q3 or KCNQ3/KCNQ5 potassium channels are the molecular basis of M currents (Wang et al., 1998; Shapiro et al., 2000; Lerche et al., 2000; Schroeder et al., 2000). Mutation of KCNQ2/3 genes results in epileptic conditions (Biervert et al., 1998; Singh et al., 1998). M/KCNQ currents are the targets of modulation of many different neurotransmitters and hormones. Activation of their corresponding receptors by a variety of neurotransmitters and hormones, such as muscarinic acetylcholine (Brown and Adams, 1980), ATP (Filippov et al., 1998; Ford et al., 2003; Zaika et al., 2007), bradykinin (Jones et al., 1995; Cruzblanca et al., 1998), angiotensin II (Shapiro et al., 1994; Zaika et al., 2006), substance P (Adams et al., 1983) and luteinizing hormone releasing hormone (LHRH) (Adams and Brown, 1980), has been shown to inhibit M/KCNQ currents. After many years of intensive effort, a clearer picture concerning the mechanism of M/KCNQ current modulation is emerging (Delmas and Brown, 2005; Suh and Hille, 2005). Neurotransmitters and peptides inhibit M/KCNQ currents through Gq/11-coupled receptors (e.g., Delmas and Brown, 2005). Three downstream signaling mechanisms following Gq/11 may directly mediate inhibition of M/KCNQ currents. These are (1) depletion of membrane PtdIns(4,5)P2 as a result of its hydrolysis (Suh and Hille, 2002; Ford et al., 2003, 2004; Zhang et al., 2003; Li et al., 2005; Winks et al., 2005); (2) an increase in intracellular Ca2+/calmodulin (Selyanko and Brown, 1996; Cruzblanca et al., 1998; Gamper and Shapiro, 2003; Gamper et al., 2005); and (3) activation of PKC (Hoshi et al., 2003; Higashida et al., 2005).
Apart from modulation by activation of G protein–coupled receptors, M/KCNQ currents are also targets of receptor (Jia et al., 2007) or nonreceptor tyrosine kinases (Gamper et al., 2003; Li et al., 2004). In the case of receptor tyrosine kinases (RTKs), activation of the epidermal growth factor receptor inhibits M/KCNQ currents through the two distinct mechanisms of membrane PtdIns(4,5)P2 hydrolysis and tyrosine phosphorylation of the channel (Jia et al., 2007).
In both human (Jentsch, 2000) and animal models (Peters et al., 2005), suppression of the neuronal M/KCNQ current is associated with epileptic conditions. M/KCNQ channel activity is thought to mediate neuronal excitability control and early spike frequency adaptation (Brown and Adams, 1980; Madison and Nicoll, 1984; Brown, 1988; Storm, 1989), and to generate afterhyperpolarizations of medium duration (mAHPs) during and after repetitive firing (Storm, 1989; Gu et al., 2005). Thus, M/KCNQ channel activity tends to stabilize the membrane potential, thereby preventing spiking. Accordingly, attenuation of M/KCNQ channel activity through activation of G protein–coupled receptors may enhance neuronal excitability responses to excitatory input (Brown, 1988; Storm, 1989; Marrion, 1997).
Neurotrophins, including NGF, regulate neuronal ion channels and membrane electrical properties in both central and peripheral neurons (Levine et al., 1995; Holm et al., 1997; Baldelli et al., 2000; Adamson et al., 2002; Zhang et al., 2002, 2006; Luther and Birren 2006). For example, NGF increases the amplitude of calcium-dependent potassium currents in cultured chick sympathetic neurons (Raucher and Dryer 1995), and regulates both calcium and sodium currents in cultured frog sympathetic ganglion B cells (Lei et al., 1997, 2001). In addition to the long-term changes in ionic currents, NGF also influences neuronal membrane electrical and firing properties on relatively short time scales. NGF treatment increases L-type calcium currents within minutes in cultured PC12 cells (Jia et al., 1999), and rapidly alters the firing properties of dorsal root ganglion sensory neurons by increasing sodium and decreasing potassium current amplitudes (Zhang et al., 2002).
Thus far there have been no documented reports concerning NGF modulation of M/KCNQ currents and the consequences of this regulation on neuronal excitability. Previous studies suggest that mammalian sympathetic neurons can be classified into two types: phasic and tonic neurons (e.g., Wang and McKinnon, 1995). It is not known whether these subpopulation neurons will respond differently to modulation. In the present study, we investigated effects of NGF on the M/KCNQ currents and excitability of superior cervical ganglion (SCG) neurons from rats. Our results demonstrate that NGF has distinct effects on the excitabilities of SCG neurons with distinct firing patterns, depending upon whether these patterns are phasic or tonic. The effects of NGF on excitability arose from its ability to inhibit M/KCNQ currents.
MATERIALS AND METHODS
Chemicals
The drugs and chemicals used in these experiments were obtained as follows. NGF, oxotremorine-M (oxo-M), linopirdine, U73122, AG879, genistein, HEPES, DMEM, and DMSO were purchased from Sigma-Aldrich Co. Tetrodotoxin (TTX) was purchased from Swellxin Science and Technology Co. Neurobasal A medium and B27 supplement were purchased from Invitrogen.
Cell Culture
Primary cultures of SCG neurons were prepared from 4- to 6-wk-old Sprague-Dawley rats using a previously described procedure (Jia et al., 2007). In brief, rats were killed by cervical dislocation and then ganglia were rapidly removed from the carotid bifurcation and placed in modified D-Hanks' solution. Ganglia were digested at 37°C with collagenase (1 mg/ml, Worthington) and dispase (5 mg/ml, Sigma-Aldrich) for 30 min, followed by another 30-min digestion with Trypsin (2.5 mg/ml, Worthington). They were subsequently suspended at least twice in DMEM medium plus 10% bovine calf serum (Hy-Clone) to stop digestion. Ganglia were then dissociated into a suspension of individual cells and plated on poly-d-lysine–coated glass coverslips in 24-well tissue culture plates (Costar). Cells were incubated at 37°C with a 5% CO2 and 95% air atmosphere. The medium was changed to Neurobasal A medium plus 2% B27 supplement (contains no NGF or other growth factors, Invitrogen) after 12 h and neurons were used for recording within 48 h. Neurons with various sizes can be seen in the cultures. Small neurons with membrane capacitances <20 pF were excluded from further experiments.
Electrophysiology
Whole-cell patch and perforated whole-cell patch recordings were used in this study. Recordings were made at room temperature (23–25°C). Pipettes were pulled from borosilicate glass capillaries and had resistances of 3–5 MΩ when filled with internal solution. Currents and action potentials were recorded using an Axon patch 200B amplifier and pClamp 9.0 software (Axon Instruments), and were filtered at 2 KHz. The protocol used to study M/KCNQ currents of SCG neurons was as follows: the cells were held at −20 mV following a 2-s hyperpolarizing step to −60 mV every 4 s. We used current-clamp method to record the action potentials of SCG neurons held at 0 and elicited the action potentials by current injection for 2 s. Solutions were applied through the VM8 fast superfusion system (ALA Instruments) or by gravity. For perforated patch recording, a pipette was first front-filled with the standard internal solution, then backfilled with the same internal solution containing amphotericin B (120 ng/ml). The external solution used to record M/KCNQ currents contained (in mM) NaCl 120, KCl 3, HEPES 5, NaHCO3 23, glucose 11, MgCl2 1.2, CaCl2 2.5, and TTX 0.00005 (adjusted to pH 7.4 with NaOH) (Tatulian et al., 2001). The internal solution for perforated patch recording consisted of (in mM) KAc 90, KCl 40, HEPES 20, and MgCl2 3 (adjusted to pH 7.3–7.4 with KOH) (Tatulian et al., 2001). Na2ATP (3 mM) and EGTA (5 mM) were added to the above internal solution for conventional whole-cell recording. The external solution used to record neuronal action potentials was the same as that used for M/KCNQ current recording, but did not contain TTX. The internal solution for action potential recording was also the same solution as that used for M/KCNQ current using perforated patch recording. In conventional whole-cell recordings, an initial rundown of M/KCNQ currents (in most cases <30%) was seen in some cells even with ATP(Mg) in the pipette. Time was allowed for rundown of M/KCNQ currents to stabilize and then effects of NGF and other modulators were studied.
For cell-attached patch recording, channel activity was recorded from cultured rat sympathetic neurons at DIV 2–4. Sylgard-coated pipettes had resistances of 8–10 MΩ when filled with a solution consisting of NaCl 125 mM, KCl 3 mM, MgCl2 1.2 mM, HEPES 10 mM, apamin 200 nM, charybdotoxin 100 nM, α and β dendrotoxins 300 nM, tetrodotoxin 250 nM, and glucose 11 mM (pH 7.3). The extracellular solution consisted of NaCl 65 mM, KCl 63 mM, CaCl2 0.5 mM, MgCl2 1.2 mM, HEPES 10 mM, CgTx-GVIA 250 nM, nifedipine 10 μM, tetrodotoxin 250 nM, and glucose 11 mM (pH 7.3 with KOH). Cells bath perfused with this high K+ solution had a resting membrane potential near −20 mV. Membrane potentials (Vm) were therefore calculated as Vm = Vrest − Vpipette, where Vrest was taken to be −20 mV and Vpipette was the voltage applied. Single-channel currents were recorded with an Axopatch 200B amplifier (Axon Instruments). Individual KCNQ/M channels studied in this work had conductance of 6.5 pS, which is similar to previous published data on native and recombinant M/KCNQ channels (6–8 pS, Selyanko and Brown, 1999; Tatulian and Brown, 2003; Li et al., 2005). They had a low threshold of activation (∼−50 mV), a steady activity at −20 mV and their activity was markedly increased when 10 μM retigabine was added to the bath (not depicted; Tatulian and Brown, 2003). The data were sampled at 5–10 kHz and filtered at 0.5–2 kHz. Transitions between open and closed states were detected by setting the threshold to 50% of the open channel level. Estimates of Po were made by analysis of 5-s recording periods. The single-channel amplitude was calculated by fitting all-point histograms with Gaussian curves for closed and open peaks. Po-Vm relationships were fitted by a Boltzmann equation of the form: Po/Po,max = 1/{1 + exp[(V1/2 − Vm)/k] }, where Po is the channel open probability, Po,max is the maximum Po obtained at Vm + 40 mV, Vm is the membrane potential, V1/2 is the half-activation potential, and k is the steepness factor.
Statistics
The programs “Origin” (Version 7.0, OriginLab Corporation) and Excel (Microsoft) were used for data analysis. Data are presented as mean ± SEM. Statistical significance was computed using Student's t test. ANOVA analysis was used for multiple comparisons. Differences are considered significant if P < 0.05.
RESULTS
Characteristics of Firing Patterns of SCG Neurons and their Relationship to M /KCNQ Currents
The major purpose of this study was to investigate the effects of NGF on M/KCNQ currents and the consequences of these effects on the neuronal excitability. It has been reported that two types of neurons differentiated by the different firing patterns of their action potentials, phasic and tonic neurons, exist in the peripheral nervous system (PNS). Previous work has shown that transient outward potassium currents (IA) and M/KCNQ currents (IM) aid in classifying PNS neurons (Cassell and McLachlan, 1986; Wang and McKinnon, 1995), and that tonic neurons lacking M/KCNQ currents are absent in rat SCG (Wang and McKinnon, 1995). To gain detailed information on the effects of NGF on neuronal excitability, we first proceeded to study the firing patterns of SCG neuron action potentials and their relationship to the M/KCNQ current present.
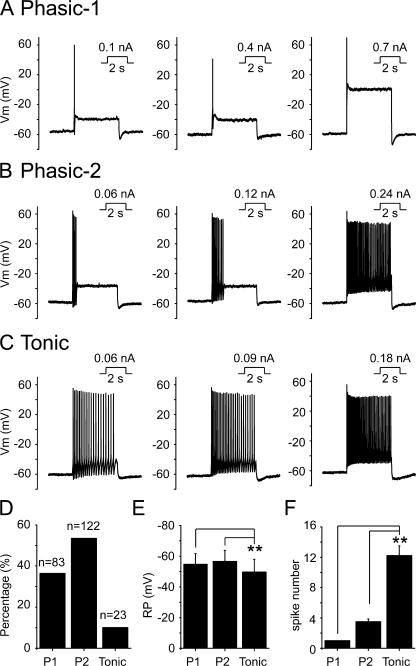
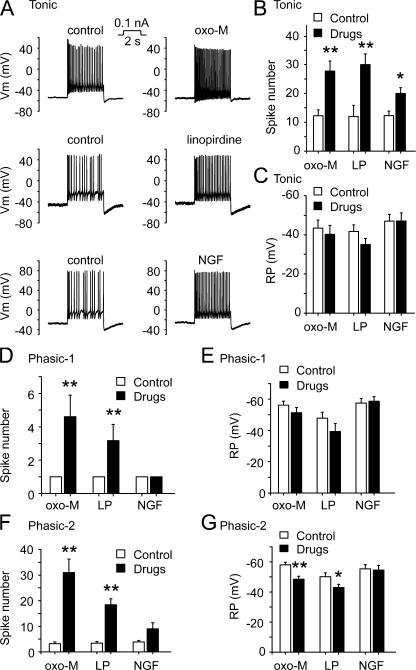
Using the perforated patch configuration, we recorded action potentials in SCG neurons with injected currents of different levels for 2 s, from a zero holding current level. Three types of neurons, namely phasic-1, phasic-2, and tonic neurons, were observed from rat SCG neurons (Fig. 1). Phasic-1 neurons, making up 36% of total SCG neurons studied (Fig. 1 D), fired only one spike during the period of stimulation even with increased current injection (Fig. 1, A and F). Phasic-2 neurons, seen in 54% of neurons (Fig. 1 D), fired two to six spikes with lesser depolarizing currents, but fired more frequently in response to the increased current injection (Fig. 1, B and F), and their phasic firing pattern eventually could be converted to a tonic firing pattern (Fig. 1 B). Tonic neurons were seen in 10% of SCG neurons (Fig. 1 D). Tonic neurons fired action potentials in a sustained manner even with a minimal stimulus, and the number of spikes increased with increased current injection (Fig. 1, C and F). When the number of spikes was compared, an approximate twofold threshold depolarizing current was used here and in the subsequent experiments. The membrane resting potential of phasic-1, phasic-2, and tonic neurons was −55 ± 7, −57 ± 7, and −50 ± 8 mV, respectively (Fig. 1 E). The difference in resting potentials between phasic-1 and phasic-2 neurons was not significant but the differences between both classes of phasic neurons and tonic neurons were significant (Fig. 1 E; P < 0.01).
Figure 1.
Classification of rat SCG neurons based on the firing patterns. (A–C) Rat SCG neurons were classified as phasic-1, phasic-2, and tonic neurons based on firing properties in response to depolarizing current stimuli. Perforated patch recording under current clamp method was used. The amplitude and the time of injected currents were shown on top of each membrane potential traces. (D) Summary data showing the percentage of phasic-1 (P1), phasic-2 (P2), and tonic neurons out of all SCG neurons tested; 36% were phasic-1 (P1), 54% were phasic-2 (P2), and 10% were tonic neurons. (E) Summary data for resting membrane potential. (F) Summary data for the number of spikes fired. **, P < 0.01 compared with tonic neurons. Error bars represent SEM.
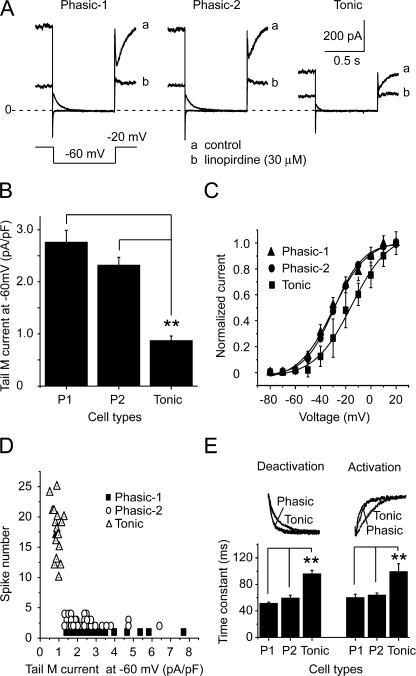
After characterizing the firing patterns of SCG neurons, we further studied the relationship between firing pattern and M/KCNQ current. For this, the firing pattern was first established, and M/KCNQ currents were then measured in the same cell. The cells were held at −20 mV, and a 2-s hyperpolarizing step to −60 mV was applied every 4 s. The amplitude of M/KCNQ currents was defined as the outward current sensitive to 30 μM linopirdine, a specific M/KCNQ channel blocker, and was measured from deactivation current records at −60 mV as the difference between the average of an initial 10-ms segment, taken 10–20 ms into the hyperpolarizing step, and the average during the last 10 ms of that step (Suh and Hille, 2002) (Fig. 2 A). The densities of the M/KCNQ tail currents for phasic-1, phasic-2, and tonic neurons were 2.8 ± 0.2, 2.3 ± 0.2, and 0.9 ± 0.1 pA/pF, respectively (Fig. 2 B). A significant difference occurred between phasic and tonic neurons (P < 0.01), but no significant difference was found between phasic-1 and phasic-2 neurons (Fig. 2 B). The membrane capacitances of phasic-1, phasic-2, and tonic neurons were 43 ± 3, 42 ± 2, and 40 ± 5 pF, respectively, and no significant differences were found among these three types of neurons. The M/KCNQ current–voltage (I-V) relationships for the three types of neurons were established (Fig. 2 C) from the tail currents at −60 mV preceded by multiple steps from −80 to +20 mV. The half-activation potentials (V1/2) for phasic-1, phasic-2, and tonic neurons were −30 ± 1, −29 ± 1, and −15 ± 3 mV, respectively (Fig. 2 C). Compared with phasic neurons, the I-V curve of tonic neurons was positively shifted (Fig. 2 C). We made a more detailed analysis of the relationship between the number of spikes fired and the density of M/KCNQ tail currents (Fig. 2 D). From this analysis, it appears that the density of M/KCNQ currents is diagnostic in separating SCG neurons into either phasic or tonic neurons. Specifically, a line of demarcation was located at an M/KCNQ current density level of ∼1 pA/pF (Fig. 2 D).
Figure 2.
Characteristics of M/KCNQ currents from phasic and tonic neurons. (A) Representative M/KCNQ current records of phasic-1, phasic-2, and tonic neurons. a is the control condition and b is after 30 μM linopirdine. M/KCNQ currents were evoked by the deactivating protocol shown under the current traces. Perforated patch method was used in these experiments. (B) Summary data for the densities of M/KCNQ current of phasic and tonic neurons. M/KCNQ currents were measured as the deactivating tail currents at −60 mV (see Materials and methods for detail). (C) The M/KCNQ tail currents at −60 mV preceded by multiple steps from −80 to +20 mV were used to produce the current–voltage (I-V) curves. The I-V relationship curves were fitted with the Boltzmann function. V1/2 for phasic-1, phasic-2, and tonic neurons was −30 ± 1 mV, −29 ± 1 mV, and −15 ± 3 mV, respectively. (D) Relationship between number of spikes fired and M/KCNQ current density. (E) Activation and deactivation of M/KCNQ currents. The top panel shows the normalized deactivating M/KCNQ currents from −20 to −60 mV (left) and the normalized activating M/KCNQ currents from −60 to −20 mV (right). The bottom panel shows the summary data for the time constants of deactivation and activation of M/KCNQ currents, which were fitted by single exponential functions. **, P < 0.01. Error bars indicate SEM.
The kinetics of M/KCNQ current activation and deactivation were also studied. Single exponential fittings were performed to obtain the time constants of activation and deactivation at −20 and −60 mV, respectively. The representative traces of these activating and deactivating currents from phasic and tonic neurons were normalized and shown in the top panel and summarized data were shown in the bottom panel of Fig. 2 E. The time constants of activation for phasic-1, phasic-2, and tonic neurons were 60 ± 5, 64 ± 4, and 99 ± 12 ms, respectively (Fig. 2 E). The time constants of deactivation for phasic-1, phasic-2, and tonic neurons were 51 ± 2, 60 ± 6, and 96 ± 5 ms, respectively (Fig. 2 E). Both deactivation and activation of tonic neurons were significantly slower than phasic neurons, but no significant differences were found between phasic-1 and phasic-2 neurons (Fig. 2 E).
NGF Inhibits M/KCNQ Currents in Rat SCG Neurons
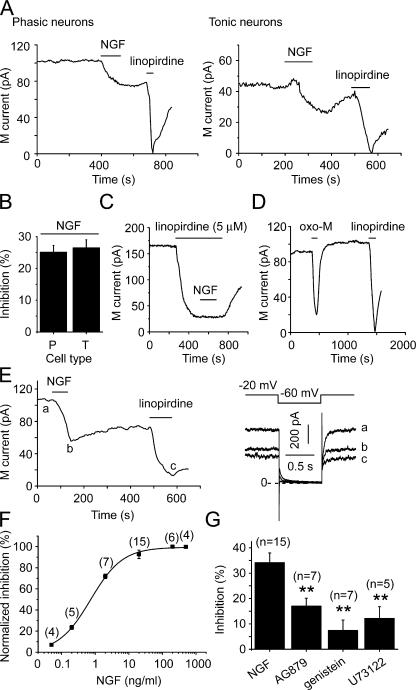
M/KCNQ currents were measured with the method described above, and the effect of NGF on M/KCNQ currents was studied. NGF (20 ng/ml) was applied to the external solution bathing the SCG neurons for 2 min. Whole-cell currents were recorded first using the perforated patch method. The effects of NGF on M/KCNQ currents from phasic and tonic neurons were compared. A relatively rapid inhibition of M/KCNQ currents was observed immediately after NGF application on both types of neurons, with average inhibition of 25 ± 2% for phasic (phasic 1 and phasic 2) and 26 ± 3% for tonic neurons (n = 10 and 6, respectively) (Fig. 3, A and B). The inhibitory effect of NGF was difficult to wash out (Fig. 3 A). To get a sense of how fast NGF acts on M/KCNQ currents, we measured time courses of NGF-induced inhibition of M/KCNQ current. The time courses can be fitted with function of single exponential decay and the constants were 103 ± 19 s (n = 8) and 102 ± 13 s (n = 5) for phasic and tonic neurons, respectively. Thus the two time courses were not significantly different. We also tested the effect of NGF on the current in presence of 5 μM linopirdine (Fig. 3 C). NGF had no further effect on the current in presence of linopirdine (Fig. 3 C, 86.3 ± 3.2% vs. 86.6 ± 3.3% inhibition, for linopirdine alone and linopirdine plus NGF, respectively, n = 7). These results suggest that the NGF-sensitive current is M-current. Oxo-M, a relatively specific muscarinic receptor agonist, was also used in these experiments. Oxo-M (10 μM) induced a reversible and large inhibition of M/KCNQ currents, with an average inhibition of 75 ± 7% (Fig. 3 D, n = 6). The effect of NGF was also studied with whole-cell recording. In this case, NGF (20 ng/ml for 2 min) inhibited M/KCNQ currents by 34 ± 4% (Fig. 3, E and G, n = 15, P < 0.01 vs. control). To get a more complete assessment of effect of NGF on M/KCNQ current, a concentration–response curve for NGF-induced inhibition of M/KCNQ current was established. NGF began to inhibit M/KCNQ current at concentration of 0.05 ng/ml and reached the maximal inhibition at concentration of 20 ng/ml (Fig. 3 F). The inhibitory effect did not significantly increase further with higher concentration of NGF (Fig. 3 F; 200 ng/ml, 37 ± 2%, n = 6; 500 ng/ml, 36 ± 1%, n = 4). The concentration–response curve was fitted by Hill equation with a half-maximal inhibition (IC50) of 0.7 ± 0.1 ng/ml, and a coefficient of 0.9 ± 0.1. We used 20 ng/ml NGF in all subsequent experiments since this is a concentration producing maximal inhibition and within the range of reported physiological concentrations of NGF in mammals (Levi-Montalcini and Calissano, 1979).
Figure 3.
NGF inhibited M/KCNQ currents from SCG neurons. (A) NGF (20 ng/ml) inhibited M/KCNQ currents from both phasic (left) and tonic (right) neurons. Time course of tail M/KCNQ currents at −60 mV was shown. Linopirdine (30 μM) was used to establish the baseline for the measurements. Perforated patch method was used in these experiments. (B) Summary data for NGF-induced inhibition of M/KCNQ currents shown in A. (C) NGF (20 ng/ml) inhibited M/KCNQ currents in presence of 5 μM linopirdine. (D) Oxo-M (10 μM) inhibited M/KCNQ currents. (E) NGF (20 ng/ml) also inhibited M/KCNQ currents recorded using conventional whole-cell method. The right panel shows the current traces taken at the times indicated in the left panel. (F) Concentration–response relationship for NGF-induced inhibition of M/KCNQ current. (G) Summary data for the effects of AG879 (50 μM, 5 min), genistein (100 μM, 5–10 min), and U73122 (3 μM, 3–5 min) on NGF-induced inhibition of M/KCNQ currents recorded using conventional whole-cell method. **, P < 0.01. Error bars represent SEM.
Our previous work demonstrated that activation of the EGF receptor inhibited KCNQ2/3/M currents through two distinct mechanisms: membrane PI(4,5)P2 hydrolysis and cellular tyrosine phosphorylation (Jia et al., 2007). The NGF receptor, like the EGF receptor, is a member of the receptor tyrosine kinase (RTK) superfamily. To explore the mechanism involved in the inhibition of M/KCNQ currents induced by NGF, we tested a series of pharmacological agents, such as AG879, genistein, and U73122. AG879 is a specific inhibitor of the Trk A receptor (Ohmichi et al., 1993; Hilborn et al., 1998), which, among two classes of NGF receptors (Trk A and p75), is a typical tyrosine kinase receptor. AG879 was included in the internal solution at a concentration of 50 μM. AG879 significantly reduced the NGF-induced inhibition of M/KCNQ currents from 34 ± 4% to 17 ± 3% (Fig. 3G, n = 7, P < 0.01), whereas it has no effect on Oxo-M–mediated inhibition of M currents (n = 7). Genistein, a broad spectrum cellular protein tyrosine kinase inhibitor, was used to pretreat SCG neurons for 5–10 min at a concentration of 100 μM. Pretreatment with genistein reduced the NGF-induced inhibition of M/KCNQ currents from 34 ± 4% to 7 ± 4% (Fig. 3 G, n = 7, P < 0.01). U73122 is a commonly used inhibitor of PLC and was used to pretreat SCG neurons for 3–5 min at a concentration of 3 μM. Pretreatment with U73122 reduced the inhibition of M/KCNQ currents produced by NGF from 34 ± 4% to 12 ± 5% (Fig. 3 G, n = 5, P < 0.01). These data suggest that NGF inhibited M/KCNQ currents through the TrkA receptor and its downstream signal pathways. It is likely that, similar to activation of the EGF receptor, activation of the NGF receptor inhibits M/KCNQ currents through both phosphorylation and PI(4,5)P2 hydrolysis (Jia et al., 2007).
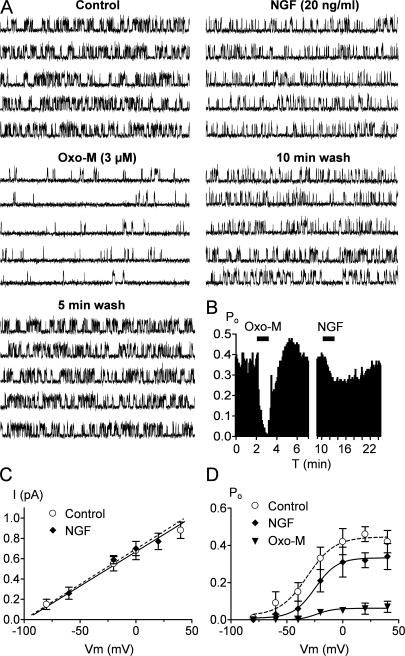
Bath-applied NGF Decreases M/KCNQ Channel Po in Cell-attached Patches
We wanted to analyze the NGF regulation of M/KCNQ channels at the single-channel level to determine whether the inhibition can be attributed to a decrease in Po or single-channel conductance. A cocktail of drugs was included in the patch pipette (see Materials and methods) to block other K+ channels (SK, BK, IKd), and transient K+ channels were inactivated at −20 mV. Criteria for identification of M/KCNQ channels were as follows: steady activity at −20 mV, single-channel conductance of 6–8 pS, and inhibition by bath-applied Oxo-M and linopirdine (10 μM) (see Materials and methods). Unitary currents were recorded using the cell-attached patch mode with 63 mM K+ in the bath to set the membrane potential near −20 mV and at a patch potential of 0 mV, which was near the maximal Po of native M/KCNQ channels (see below). A representative experiment from a patch containing a single M/KCNQ channel is shown in Fig. 4 A. Unitary currents were studied before, during, and after bath application of Oxo-M (3 μM) and NGF (20 ng/ml). In this patch, Po was 0.36 in the control, slightly higher than that classically observed for KCNQ2/Q3 channels in heterologous systems (Li et al., 2005). This may be due to our recording conditions, which were selected to set [Ca2+]i at low levels (Selyanko and Brown, 1996). Bath application of Oxo-M strongly reduced M/KCNQ channel Po within ∼1 min to 0.02 (Fig. 4, A and B). After washout of Oxo-M effects, cell exposure to NGF reduced channel Po by 28% from 0.39 to 0.28, reaching a maximal inhibition within 4–6 min (Fig. 4, A and B). Channel activity was only partly reversible to a Po of 0.36 11 min after washout of NGF. In seven such patches tested, the channel Po of the control was inhibited by 89 ± 2% and 29 ± 3% after application of Oxo-M and NGF, respectively. These data are consistent with our whole-cell experiments described above.
Figure 4.
Single-channel analysis of NGF action. (A) Single-channel records at 0 mV from a cell-attached patch containing one KCNQ/M channel, before, during, and after bath application of 3 μM Oxo-M and 20 ng/ml NGF. Five consecutive 1-s sweep are shown for each condition. Bars: 100 ms, 1 pA. (B) The time course of Po plotted against time for the experiment shown in A. Po calculated from 5-s periods. The application of Oxo-M and NGF are indicated by the horizontal bars. (C) Single-channel amplitudes derived from all point amplitude histograms were plotted against membrane potentials. The mean slope conductance obtained by fitting a linear regression was 6.5 ± 0.2 and 6.1 ± 0.2 pS (n = 5) in the presence and absence of NGF, respectively. (D) Mean Po-Vm curves determined in control and in the presence of NGF or Oxo-M. Data points were fitted by a Boltzmann equation, yielding values for Po,max, V1/2, and k of (control, ○) 0.44 ± 0.02, −32 ± 3 mV, 12 ± 2 mV; (NGF, ♦) 0.33 ± 0.08, −25 ± 2 mV, 10 ± 2 mV; (Oxo-M, ▾) 0.06 ± 0.005, −29 ± 3 mV, 8 ± 2 mV, respectively. Each data point is the mean ± SEM of 5–7 patches. Error bars show SEM.
We also investigated whether NGF altered the single-channel conductance of M/KCNQ channels. Single-channel amplitudes were determined using all point amplitude histograms over a range of potentials both in the presence and absence of NGF. NGF did not alter M/KCNQ single-channel conductance, which was 6.5 ± 0.2 and 6.1 ± 0.2 pS (n = 5) in the presence and absence of NGF, respectively (Fig. 4 C). Oxo-M also had no effect on M/KCNQ single-channel conductance (6.4 ± 0.3 pS, n = 7). From such experiments, we constructed Po-Vm relationships before and during application of NGF (Fig. 4 D). Boltzmann fits to the data points revealed a reduction in Po at all voltages for both NGF and Oxo-M. In addition, NGF, but not Oxo-M, shifted the M/KCNQ channel V1/2 from −32 ± 3 to −25 ± 2 mV (Fig. 4 D).
NGF Increased the Excitability of Tonic Neurons but Not Phasic Neurons
We tested the effects of NGF on the excitability of the three different types of SCG neurons. NGF (20 ng/ml, ∼2 min) significantly increased the number of spikes fired in tonic neurons from 12 ± 2 to 20 ± 2 (Fig. 5, A and B, n = 9, P < 0.05). The resting membrane potential of tonic neurons was not significantly changed by NGF (−47 ± 3 and −47 ± 4 mV, before and after NGF, respectively) (Fig. 5 C). In the same batch of tonic neurons, both Oxo-M (10 μM) and linopirdine (30 μM) rapidly and significantly enhanced the number of spikes fired from 12 ± 2 and 12 ± 5 to 28 ± 4 and 30 ± 4, respectively (Fig. 5 B, n = 8 and 7, P < 0.01). Small depolarization from the resting membrane potentials was seen with applications of Oxo-M and linopirdine, but the changes did not reach statistical significance (Fig. 5 C).
Figure 5.
NGF enhanced firing of tonic neurons, whereas Oxo-M and linopirdine enhanced both tonic and phasic neuronal excitability. (A) Effects of Oxo-M (10 μM, ∼1 min), linopirdine (30 μM, ∼1 min), and NGF (20 ng/ml, ∼2 min) on firing of action potentials from tonic neurons. Perforated patch method was used in these experiments. (B and C) Summary data for number of spikes fired and resting membrane potential of tonic neurons, respectively. (D and E) Summary data for number of spikes fired and resting membrane potential of phasic-1 neurons, respectively. (F and G) Summary for number of spikes fired and resting membrane potential of phasic-2 neurons, respectively. LP is abbreviation of linopirdine. *, P < 0.05; **, P < 0.01. Error bars indicate SEM.
NGF (20 ng/ml) did not alter the one-spike firing property of phasic-1 neurons (Fig. 5 D); NGF (20 ng/ml) increased the number of spikes of phasic-2 neurons from 3.9 ± 0.6 to 9 ± 2.4 but the difference did not reach statistical significance (Fig. 5 F). Similarly resting membrane potentials were not significantly changed by NGF (Fig. 5, E and G). On the other hand, Oxo-M (10 μM) and linopirdine (30 μM) significantly increased the number of spikes fired (Fig. 5, D and F) in both phasic-1 and phasic-2 neurons. Oxo-M (10 μM) increased the spike numbers of phasic-1 and phasic-2 neurons from 1 and 3.2 ± 0.7 to 4.7 ± 1.2 (n = 6, P < 0.01) and 31 ± 6 (n = 8, P < 0.01), respectively (Fig. 5, D and F); linopirdine (30 μM) increased the number of spikes fired in phasic-1 and phasic-2 neurons from 1 and 3.1 ± 0.5 to 3.2 ± 1 (n = 6, P < 0.01) and 16 ± 2 (n = 9, P < 0.01), respectively (Fig. 5, D and F). Both agents depolarized the resting membrane potentials in both phasic neuron classes (Fig. 5, E and G), but these effects reached a statistically significant level only in phasic-2 neurons (from −58 ± 2 to −49 ± 2 mV for Oxo-M, and from −50 ± 3 to −43 ± 2 mV for linopirdine, respectively; Fig. 5 G). Thus, Oxo-M and linopirdine, which resulted in greater inhibition of M currents than NGF, increased excitability of all three types of SCG neurons, whereas NGF only increased excitability of tonic neurons. These results indicate that under physiological conditions, NGF may specifically, but not universally, modulate neuronal excitability.
We went further to study if Oxo-M and NGF inhibit M/KCNQ currents and alter the excitability of SCG neurons through a similar mechanism. Considering that Oxo-M is a stronger modulator than NGF of both M/KCNQ currents and excitability, a similar mechanism of action would confer upon Oxo-M the ability to exclude any further effect from NGF and to have a further effect on top of that of NGF.
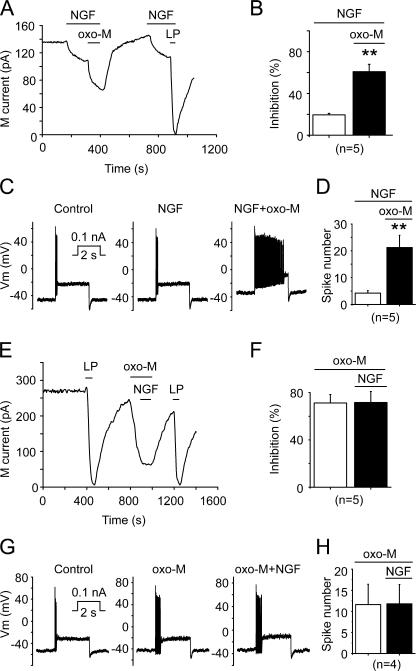
As shown before, NGF (20 ng/ml) inhibited M/KCNQ currents by 20 ± 2% (Fig. 6, A and B, n = 5, P < 0.01). Oxo-M (10 μM) in the presence of NGF inhibited M/KCNQ currents by 62 ± 7% (Fig. 6, A and B, n = 5, P < 0.01). Under these circumstance, NGF (20 ng/ml) did not alter the excitability of phasic neurons, and a significant enhancement of the excitability was produced by Oxo-M (10 μM) in the presence of NGF (Fig. 6, C and D; n = 5, P < 0.01); Oxo-M increased the number of spikes fired in phasic neurons from 4.4 ± 0.7 to 22 ± 5 in the presence of NGF (Fig. 6 D). In a similar experiment but with reversed sequential applications of the drugs, Oxo-M (10 μM) inhibited M/KCNQ currents by 72 ± 8% (n = 5, P < 0.01); following application of NGF (20 ng/ml) in the presence of Oxo-M, no further inhibition of M/KCNQ currents was seen (Fig. 6, E and F). Oxo-M (10 μM) alone significantly increased the number of spikes fired in phasic-2 neurons from 3.4 ± 1.2 to 12 ± 5 (Fig. 6, G and H; n = 5, P < 0.01 vs. control), and a subsequent application of NGF (20 ng/ml) did not show a significant effect (Fig. 6, G and H).
Figure 6.
The effects of coapplication of NGF and Oxo-M on M/KCNQ currents and neuronal excitability. (A) Oxo-M (10 μM, 1 min) inhibited M/KCNQ currents further on top of inhibition induced by NGF (20 ng/ml, 2 min). M/KCNQ current was recorded from a phasic neuron and the time course of M/KCNQ currents recorded at −60 mV was shown. Perforated patch method was used in these experiments. (B) Summary data for experiments as in A. (C) NGF (20 ng/ml, ∼2 min) did not affect, and subsequent application of oxo-M (10 μM, 1 min) increased firing of action potentials from a phasic neuron. (D) Summary data for experiments as in C. (E) Oxo-M (10 μM, 1 min) inhibited M/KCNQ current and occluded further inhibition by NGF (20 ng/ml, 2 min) in a phasic neuron. (F) Summary data for experiments as in E. (G) Oxo-M (10 μM, ∼1 min) increased, and subsequent application of NGF (20 ng/ml, ∼2 min) did not affect firing of action potentials from a phasic neuron. (H) Summary data for experiments as in G. **, P < 0.01. Error bars indicate SEM.
Small Inhibition of M/KCNQ Currents by Low Concentrations of Linopirdine Mimics the Effects of NGF on Neuronal Excitability
The inability of NGF to modulate the excitability of phasic neurons could be due to an insufficient inhibition of the large M/KCNQ current present in these neurons. It has been reported that ∼25% reduction in KCNQ2/3 function (Schroeder et al., 1998) is sufficient to cause the electrical hyperexcitability in BFNC (benign familial neonatal convulsion). Thus, it is worthwhile to study whether a mere manipulation of M/KCNQ currents correlates well with alterations of neuronal excitability. For this purpose, we choose linopirdine to study the correlation between M/KCNQ currents and excitability in SCG neurons, simply because linopirdine is a specific M/KCNQ current blocker that is devoid of the complications from cell signaling involvement present with both oxo-M and NGF.
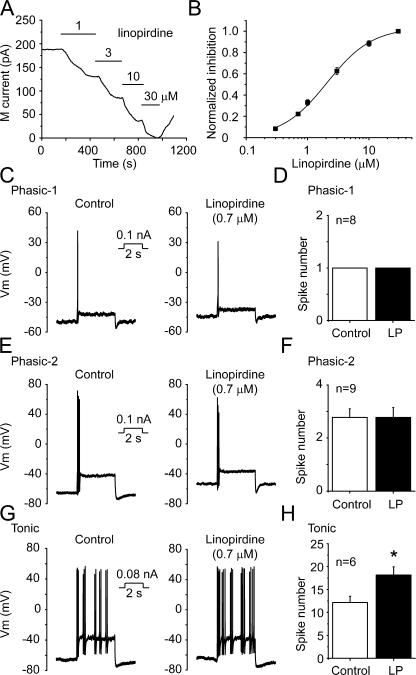
We first wanted to find the proper concentration of linopirdine that would produce an inhibitory effect on M/KCNQ currents similar to that seen with NGF. For this, a concentration–response curve was generated for linopirdine-induced inhibition of M/KCNQ currents. Linopirdine (1–30 μM) inhibited M/KCNQ currents in a concentration-dependent manner (Fig. 7 A). Linopirdine began to inhibit M/KCNQ currents at concentrations as low as 0.3 μM, and reached maximal inhibition at 30 μM (Fig. 7 B). The concentration–response curve of linopirdine was fitted by the Hill function with a concentration for half-maximum inhibition (IC50) of 2.1 ± 0.2 μM and a coefficient of 1.2 ± 0.1 (Fig. 7 B). According to the concentration–response curve, 0.7 μM linopirdine would inhibit M/KCNQ currents by 25%, a degree of inhibition similar to that produced by NGF.
Figure 7.
Linopirdine at low concentrations mimicked NGF action on neuronal excitability. (A) The time course of M/KCNQ currents inhibited by different concentrations of linopirdine. The record is from a phasic neuron and the M/KCNQ current was recorded at −60 mV as described before. Perforated patch method was used in these experiments. (B) Concentration–response curve for linopirdine-induced inhibition of M/KCNQ currents fitted with the Hill function. IC50 for linopirdine is 2.1 ± 0.2 μM, and the coefficient is 1.2 ± 0.1 (C, E, and G) The effects of linopirdine at 0.7 μM (LP, 0.7 μM) on firing of action potential from phasic-1, phasic-2, and tonic neurons, respectively. (D, F, and H) Summary data for C, E, and G, respectively. *, P < 0.05. Error bars indicate SEM.
At a concentration of 0.7 μM, linopirdine did not affect the excitability of phasic-1 neurons (Fig. 7, C and D). Similarly, linopirdine did not change the spike numbers of phasic-2 neurons at this concentration (Fig. 7, E and F). On the other hand, 0.7 μM linopirdine significantly increased the number of spikes fired in tonic neurons from 12 ± 1 to 18 ± 2 (Fig. 7, G and H; n = 6, P < 0.05). This effect of linopirdine at a low concentration mimicked that of NGF on tonic neurons (Fig. 5, A and B; Fig. 7, G and H). Thus, the selective modulation of excitability of tonic neurons by NGF is likely due to its moderate capability of inhibiting M/KCNQ currents.
DISCUSSION
In the present study, we have demonstrated that NGF inhibits M/KCNQ currents from rat SCG neurons. However, even at maximal concentrations, NGF was much less potent than Oxo-M, an agonist of muscarinic receptors, in inhibiting M/KCNQ currents. The characteristic inhibition of M/KCNQ currents by NGF was manifested by a selective potentiation of excitability in tonic SCG neurons. Thus, NGF can now be added to the expanding family of identified M/KCNQ modulators and may have important physiological implications.
NGF may inhibit M/KCNQ currents through mechanisms similar to those employed by activation of the muscarinic receptor (Zhang et al., 2003) and the EGF receptor (Jia et al., 2007). Activation of the muscarinic receptor inhibits M/KCNQ currents through depletion of membrane PtdIns(4,5)P2 resulting from hydrolysis (Zhang et al., 2003); activation of the EGF receptor inhibits M/KCNQ currents through both depletion of membrane PtdIns(4,5)P2 and channel tyrosine phosphorylation (Jia et al., 2007). In both whole-cell (Fig. 3) and cell-attached patch (Fig. 4) recordings, NGF, at a near maximal concentration of 20 ng/ml, was less potent than Oxo-M (an agonist of the muscarinic receptor) in inhibiting M/KCNQ currents. Following this line, the inhibition of M/KCNQ currents by Oxo-M excluded further inhibition by NGF, whereas Oxo-M produced further inhibition on top of NGF-induced inhibition (Fig. 6). One possible explanation for these results would be that NGF and Oxo-M inhibit M/KCNQ currents through a common mechanism. We have, in our previous work, characterized EGF-induced inhibition of M/KCNQ currents (Jia et al., 2007). The NGF receptor and the EGF receptor belong to the same RTK family and trigger common signal transduction mechanisms (Hubbard, 1999; Schlessinger, 2000). Pharmacological studies, used in our previous study of EGF (Jia et al., 2007) and in this study (Fig. 3 G), strongly support the idea that NGF inhibits M/KCNQ currents through a mechanism similar to EGF. The results from cell-attached patch experiments are interesting (Fig. 4). A cytoplasmic diffusible second messenger mechanism would be an immediate consideration for both NGF- and Oxo-M–induced inhibition of M/KCNQ currents with this type of experiment. However, lateral membrane PtdIns(4,5)P2 diffusion is a preferable explanation (Zhang et al., 2003); in this case, NGF- and Oxo-M–induced hydrolysis of membrane PtdIns(4,5)P2 outside of the recoding pipette would promote diffusing out of PtdIns(4,5)P2 in the membrane isolated by the recording pipette. For NGF, an activated diffusible tyrosine kinase might also contribute to the observed inhibition of M/KCNQ currents.
Previous studies suggest that mammalian sympathetic neurons can be classified into two types: phasic and tonic neurons (Weems and Szurszewski, 1978; Decktor and Weems, 1983; Cassell et al., 1986; King and Szurszewski, 1988; Wang and McKinnon, 1995). We classified rat SCG neurons into three types based on their firing properties. Phasic-1 neurons fired only one action potential in response to a long-lasting excitatory current, and the spike number did not increase with increasing current stimuli (Fig. 1 A). Phasic-2 neurons fired a transient discharge of action potentials in response to a long-lasting excitatory current, and the spike number increased and converted to tonic firing upon receiving increasing excitatory current (Fig. 1 B). Tonic neurons fired a sustained train of action potentials in response to a small current stimulus, and the spike numbers increased in response to increasing current stimuli (Fig. 1 C). It should be noted that some of the tonic neurons fire at a relative constant rate (e.g., Fig. 1 C; Fig. 5 A top), whereas others fire in an intermittent pattern (e.g., Fig. 5 A, middle and bottom; Fig. 7 G). It is not clear what underlies the difference but both types of neurons were regarded as tonic neurons. Previous studies gave different results regarding the types of neurons presented in SCG. Some studies show that all neurons in SCG are phasic neurons (Wang and McKinnon, 1995; Jobling and Gibbins, 1999). Others have reported that all neurons in neonatal rat SCG are tonic ones (Luther and Birren, 2006), or that phasic and tonic neurons are at a ratio of 43%:57% in embryonic and postnatal 1-d rat SCG (according to the standard of classification, adapting neurons are tonic neurons) (Malin and Nerbonne, 2000). The exact cause for such apparent discrepancies is not clear, but the differences in animal age in these experiments are one possibility. It is clear from this study that M/KCNQ current level present in 4–6-wk rat SCG neurons are among the crucial factors in determining the firing properties of these neurons. Thus, neurons with small M/KCNQ currents will fire more easily (tonic neurons) and neurons with higher expression of M/KCNQ currents will be more difficult to excite (phasic neurons) (Fig. 2; Wang and McKinnon, 1995). Although the mRNA levels of KCNQ2 and KCNQ5 in rat SCG neurons are stable between the embryonic stage (18/19 d) and the young adult (postnatal 45 d), the mRNA level of KCNQ3 is increased after the postnatal stage (Hadley et al., 2003). This increase would be expected to increase the heteromeric currents formed from KCNQ2, KCNQ3, and KCNQ5, the basis for M currents. Therefore, it is not surprising that most neurons in embryonic and neonatal rat SCG are tonic neurons (Malin and Nerbonne, 2000; Luther and Birren, 2006), whereas most neurons in young adult rat SCG are phasic neurons (Fig. 1; Wang and McKinnon, 1995). Previous work by Wang and McKinnon (1995) also classified phasic neurons into phasic-1 and phasic-2 neurons. However, they described all SCG neurons as phasic neurons and did not show neurons with tonic-firing property. Furthermore, they reported that 95% of SCG neurons were phasic-1 neurons, and phasic-2 neurons would not fire tonically even for large stimuli. The major difference between their study and the current work is that they used intracellular recordings from excised ganglion whereas we used patch clamp from SCG neurons in short-term culture.
Luther and Birren (2006) studied the effect of NGF on excitability of rat SCG neurons in an earlier work. They found that NGF reduced the total number of spikes fired but increased firing frequency, demonstrating an NGF-dependent change from a tonic to a phasic firing pattern. They attributed this NGF effect to NGF-induced inhibition of a number of K+ currents (Ca2+ dependent and Ca2+ independent). In their study, the effect of NGF was not separately studied in subpopulations of SCG neurons as we did in this current study. Our results show NGF increased the number of spikes fired in SCG neurons, more significantly in tonic neurons and did not affect the firing of phasic-1 neurons. It is likely that effect of NGF on excitability is due to its inhibition on M/KCNQ currents. Unexpectedly, NGF, unlike Oxo-M, only increased the firing of action potentials in tonic neurons without affecting the firing of phasic neurons. This difference was not caused by a different efficacy in inhibiting M/KCNQ currents by NGF in these two types of neurons because NGF inhibited M/KCNQ currents in these two neurons similarly (Fig. 3, A and B). The simplest explanation is that whereas a moderate inhibition of M/KCNQ currents by NGF in tonic neurons (which have small intrinsic M/KCNQ currents) is large enough to increase neuronal excitability, a similar proportional reduction of M/KCNQ currents in phasic neurons still leaves large enough M/KCNQ currents to dampen the firing of action potentials. On the other hand, both Oxo-M and linopirdine inhibit M/KCNQ currents much more significantly than NGF (Fig. 3 D), they should increase the firing of action potentials in both tonic and phasic neurons (Fig. 5). This working hypothesis is confirmed by results shown in Fig. 7. In this experiment, linopirdine, at a concentration that produced an inhibition of M/KCNQ currents similar to that seen with NGF, also only increased firing of tonic neurons, but not phasic neurons.
These results prompt some interesting thoughts. It has been reported that a moderate loss (∼25%) of KCNQ2/Q3 function due to channel mutation will lead to epileptic conditions in newborn infants (BFNC; Schroeder et al., 1998). Based on the data shown in Fig. 2 B, a 25% loss from 2.8 pA/pF (phasic 1) or 2.3 pA/pF (phasic 2) leaves >1.7 pA/pF of current in phasic neurons and this is still well above the 0.9 pA/pF of tonic neurons. Apparently 1.7 pA/pF suffices to maintain the phasic nature (Fig. 2 D). This would imply that the diseased neurons should be tonic-type neurons that express small M/KCNQ currents and that their excitability is sensitive to a small (25%) reduction in current amplitude. This could well be the case. Embryonic and neonatal neurons are normally found to be tonic neurons (Malin and Nerbonne, 2000; Luther and Birren, 2006). In mammalian CNS neurons, the density of M/KCNQ currents is possibly at a low level (Brown and Yu, 2000). For example, in pyramidal neurons acutely isolated from rat cerebral cortex, the M/KCNQ currents are small (Nishikawa et al., 1994). In primary cultured mouse cortical neurons, M/KCNQ currents are not detectable (Yu et al., 1997). The BFNC phenotype is characterized by frequent seizures starting in the first week of life. In most cases, the seizures spontaneously disappear within weeks. This may coincide with an increased expression of M/KCNQ currents with development, as indicated by the increased expression of KCNQ3 mRNA (Hadley et al., 2003). However, a detailed well-controlled comparative study on M/KCNQ currents from different origin of neurons and from different stages of development is lacking, and this is surely an interesting issue to address in the future.
Apart from the well-known effects of NGF and other neurotrophin (NT) members on neuronal cell survival, proliferation, and differentiation (Poo, 2001; Chao, 2003), evidence is accumulating that neurotrophins, including NGF, regulate neuronal ion channels and membrane electrical properties in both central and peripheral neurons (e.g., Holm et al., 1997; Baldelli et al., 2000; Adamson et al., 2002; Zhang et al., 2002, 2006; Luther and Birren, 2006). It has been suggested that NGF can modulate neuronal excitability in a neurotransmitter-like manner, although NGF acts over a relatively long time course (Zhang et al., 2002; 2006). We found in the present study that NGF acutely inhibited M/KCNQ currents, and this inhibition resulted in a selective enhancement of the excitability of tonic neurons. Since we used a physiological concentration of NGF, this selective modification of neuronal excitability by NGF may be physiologically relevant. Phasic neurons receive one or a few large, suprathreshold synaptic inputs from preganglionic motor neurons (Skok and Ivanov, 1983; Hirst and McLachlan, 1986) and then function as relay neurons in transmitting the information from the central nervous system to peripheral organs or tissues. On other hand, tonic neurons receive multiple small, subthreshold synaptic inputs that have to summate in order to fire the cell (Crowcroft et al., 1971; McLachlan and Meckler, 1989). Thus, a selective modification of the excitability of tonic neurons by NGF would selectively affect tonic-mediated physiological signals.
Acknowledgments
This work was supported by a NSFC grant (30730031), grant from the Ministry of Science and Technology of China (2007CB512100), a National 863 project (2006AA02Z183) (to H. Zhang), a Hebei Nature Science Foundation grant (C200700829) to Q. Jia, and by the Centre National de la Recherche Scientifique (CNRS) and grants from the Agence Nationale de la Recherche (ANR) and the Foundation pour la Recherche Médicale (to P. Delmas). H. Zhang is a beneficiary of the National Science Fund for Distinguished Young Scholars of China (30325038).
Olaf S. Andersen served as editor.
Z. Jia and J. Bei contributed equally to this work.
Abbreviations used in this paper: BFNC, benign familial neonatal convulsion; NGF, nerve growth factor; RTK, receptor tyrosine kinase; SCG, superior cervical ganglion; TTX, tetrodotoxin.
References
- Adams, P.R., and D.A. Brown. 1980. Luteinizing hormone-releasing factor and muscarinic agonists act on the same voltage-sensitive K+-current in bullfrog sympathetic neurones. Br. J. Pharmacol. 68:353–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, P.R., D.A. Brown, and S.W. Jones. 1983. Substance P inhibits the M-current in bullfrog sympathetic neurones. Br. J. Pharmacol. 79:330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson, C.L., M.A. Reid, and R.L. Davis. 2002. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J. Neurosci. 22:1385–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli, P., P.E. Forni, and E. Carbone. 2000. BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur. J. Neurosci. 12:4017–4032. [DOI] [PubMed] [Google Scholar]
- Biervert, C., B.C. Schroeder, C. Kubisch, S.F. Berkovic, P. Propping, T.J. Jentsch, and O.K. Steinlein. 1998. A potassium channel mutation in neonatal human epilepsy. Science. 279:403–406. [DOI] [PubMed] [Google Scholar]
- Brown, B.S., and S.P. Yu. 2000. Modulation and genetic identification of the M channel. Prog. Biophys. Mol. Biol. 73:135–166. [DOI] [PubMed] [Google Scholar]
- Brown, D.A. 1988. M-currents: an update. Trends Neurosci. 11:294–299. [DOI] [PubMed] [Google Scholar]
- Brown, D.A., and P.R. Adams. 1980. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 283:673–676. [DOI] [PubMed] [Google Scholar]
- Cassell, J.F., A.L. Clark, and E.M. McLachlan. 1986. Characteristics of phasic and tonic sympathetic ganglion cells of the guinea-pig. J. Physiol. 372:457–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell, J.F., and E.M. McLachlan. 1986. The effect of a transient outward current (IA) on synaptic potentials in sympathetic ganglion cells of the guinea-pig. J. Physiol. 374:273–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, M.V. 2003. Neurotrophins and their receptors: a convergence point for many signaling pathways. Nat. Rev. Neurosci. 4:299–309. [DOI] [PubMed] [Google Scholar]
- Crowcroft, P.J., M.E. Holman, and J.H. Szurszewski. 1971. Excitatory input from the distal colon to the inferior mesenteric ganglion in the guinea-pig. J. Physiol. 219:443–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzblanca, H., D.S. Koh, and B. Hille. 1998. Bradykinin inhibits M current via phospholipase C and Ca2+ release from IP3-sensitive Ca2+ stores in rat sympathetic neurons. Proc. Natl. Acad. Sci. USA. 95:7151–7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decktor, D.L., and W.A. Weems. 1983. An intracellular characterization of neurones and neural connexions within the left coeliac ganglion of cats. J. Physiol. 341:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas, P., and D.A. Brown. 2005. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat. Rev. Neurosci. 6:850–862. [DOI] [PubMed] [Google Scholar]
- Filippov, A.K., T.E. Webb, E.A. Barnard, and D.A. Brown. 1998. P2Y2 nucleotide receptors expressed heterologously in sympathetic neurons inhibit both N-type Ca2+ and M-type K+ currents. J. Neurosci. 18:5170–5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, C.P., P.L. Stemkowski, P.E. Light, and P.A. Smith. 2003. Experiments to test the role of phosphatidylinositol 4,5-bisphosphate in neurotransmitter-induced M-channel closure in bullfrog sympathetic neurons. J. Neurosci. 23:4931–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, C.P., P.L. Stemkowski, and P.A. Smith. 2004. Possible role of phosphatidylinositol 4, 5 bisphosphate in luteinizing hormone releasing hormone-mediated M-current inhibition in bullfrog sympathetic neurons. Eur. J. Neurosci. 20:2990–2998. [DOI] [PubMed] [Google Scholar]
- Gamper, N., and M.S. Shapiro. 2003. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J. Gen. Physiol. 122:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper, N., J.D. Stockand, and M.S. Shapiro. 2003. Subunit-specific modulation of KCNQ potassium channels by Src tyrosine kinase. J. Neurosci. 23:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper, N., Y. Li, and M.S. Shapiro. 2005. Structural requirements for differential sensitivity of KCNQ K+ channels to modulation by Ca2+/calmodulin. Mol. Biol. Cell. 16:3538–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, N., K. Vervaeke, H. Hu, and J.F. Storm. 2005. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J. Physiol. 566:689–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley, J.K., G.M. Passmore, L. Tatulian, M. Al-Qatari, F. Ye, A.D. Wickenden, and D.A. Brown. 2003. Stoichiometry of expressed KCNQ2/KCNQ3 potassium channels and subunit composition of native ganglionic M channels deduced from block by tetraethylammonium. J. Neurosci. 23:5012–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell, J.V., and P.R. Adams. 1982. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 250:71–92. [DOI] [PubMed] [Google Scholar]
- Higashida, H., N. Hoshi, J.S. Zhang, S. Yokoyama, M. Hashii, D. Jin, M. Noda, and J. Robbins. 2005. Protein kinase C bound with A-kinase anchoring protein is involved in muscarinic receptor-activated modulation of M-type KCNQ potassium channels. Neurosci. Res. 51:231–234. [DOI] [PubMed] [Google Scholar]
- Hilborn, M.D., R.R. Vaillancourt, and S.G. Rane. 1998. Growth factor receptor tyrosine kinases acutely regulate neuronal sodium channels through the src signaling pathway. J. Neurosci. 18:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst, G.D., and E.M. McLachlan. 1986. Development of dendritic calcium currents in ganglion cells of the rat lower lumbar sympathetic chain. J. Physiol. 377:349–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, N.R., P. Christophersen, S.P. Olesen, and S. Gammeltoft. 1997. Activation of calcium-dependent potassium channels in mouse [correction of rat] brain neurons by neurotrophin-3 and nerve growth factor. Proc. Natl. Acad. Sci. USA. 94:1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi, N., J.S. Zhang, M. Omaki, T. Takeuchi, S. Yokoyama, N. Wanaverbecq, L.K. Langeberg, Y. Yoneda, J.D. Scott, D.A. Brown, and H. Higashida. 2003. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat. Neurosci. 6:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard, S.R. 1999. Structural analysis of receptor tyrosine kinases. Prog. Biophys. Mol. Biol. 71:343–358. [DOI] [PubMed] [Google Scholar]
- Jentsch, T.J. 2000. Neuronal KCNQ potassium channels: physiology and role in disease. Nat. Rev. Neurosci. 1:21–30. [DOI] [PubMed] [Google Scholar]
- Jia, M., M. Li, X.W. Liu, H. Jiang, P.G. Nelson, and G. Guroff. 1999. Voltage-sensitive calcium currents are acutely increased by nerve growth factor in PC12 cells. J. Neurophysiol. 82:2847–2852. [DOI] [PubMed] [Google Scholar]
- Jia, Q., Z. Jia, Z. Zhao, B. Liu, H. Liang, and H. Zhang. 2007. Activation of epidermal growth factor receptor inhibits KCNQ2/3 current through two distinct pathways: membrane PtdIns(4,5)P2 hydrolysis and channel phosphorylation. J. Neurosci. 27:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling, P., and I.L. Gibbins. 1999. Electrophysiological and morphological diversity of mouse sympathetic neurons. J. Neurophysiol. 82:2747–2764. [DOI] [PubMed] [Google Scholar]
- Jones, S., D.A. Brown, G. Milligan, E. Willer, N.J. Buckley, and M.P. Caulfield. 1995. Bradykinin excites rat sympathetic neurons by inhibition of M current through a mechanism involving B2 receptors and G αq/11. Neuron. 14:399–405. [DOI] [PubMed] [Google Scholar]
- King, B.F., and J.H. Szurszewski. 1988. Electrotonic characteristics and membrane properties of neurons in the inferior mesenteric ganglion in guinea-pig. J. Auton. Nerv. Syst. 23:229–239. [DOI] [PubMed] [Google Scholar]
- Lei, S., W.F. Dryden, and P.A. Smith. 1997. Regulation of N- and L-type Ca2+ channels in adult frog sympathetic ganglion B cells by nerve growth factor in vitro and in vivo. J. Neurophysiol. 78:3359–3370. [DOI] [PubMed] [Google Scholar]
- Lei, S., W.F. Dryden, and P.A. Smith. 2001. Nerve growth factor regulates sodium but not potassium channel currents in sympathetic B neurons of adult bullfrogs. J. Neurophysiol. 86:641–650. [DOI] [PubMed] [Google Scholar]
- Lerche, C., C.R. Scherer, G. Seebohm, C. Derst, A.D. Wei, A.E. Busch, and K. Steinmeyer. 2000. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J. Biol. Chem. 275:22395–22400. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini, R., and P. Calissano. 1979. The nerve-growth factor. Sci. Am. 240:68–77. [DOI] [PubMed] [Google Scholar]
- Levine, E.S., C.F. Dreyfus, I.B. Black, and M.R. Plummer. 1995. Differential effects of NGF and BDNF on voltage-gated calcium currents in embryonic basal forebrain neurons. J. Neurosci. 15:3084–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., N. Gamper, D.W. Hilgemann, and M.S. Shapiro. 2005. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol (4,5)-bisphosphate. J. Neurosci. 25:9825–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., P. Langlais, N. Gamper, F. Liu, and M.S. Shapiro. 2004. Dual phosphorylations underlie modulation of unitary KCNQ K+channels by Src tyrosine kinase. J. Biol. Chem. 279:45399–45407. [DOI] [PubMed] [Google Scholar]
- Luther, J.A., and S.J. Birren. 2006. Nerve growth factor decreases potassium currents and alters repetitive firing in rat sympathetic neurons. J. Neurophysiol. 96:946–958. [DOI] [PubMed] [Google Scholar]
- Madison, D.V., and R.A. Nicoll. 1984. Control of the repetitive discharge of rat CA1 pyramidal neurones in vitro. J. Physiol. 354:319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin, S.A., and J.M. Nerbonne. 2000. Elimination of the fast transient in superior cervical ganglion neurons with expression of KV4.2W362F: molecular eissection of IA. J. Neurosci. 20:5191–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion, N.V. 1997. Control of M current. Annu. Rev. Physiol. 59:483–504. [DOI] [PubMed] [Google Scholar]
- McLachlan, E.M., and R.L. Meckler. 1989. Characteristics of synaptic input to three classes of sympathetic neurone in the coeliac ganglion of the guinea-pig. J. Physiol. 415:109–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, M., M. Munakata, and N. Akaike. 1994. Muscarinic acetylcholine response in pyramidal neurones of rat cerebral cortex. Br. J. Pharmacol. 112:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmichi, M., L. Pang, V. Ribon, A. Gazit, A. Levitzki, and A.R. Saltiel. 1993. The tyrosine kinase inhibitor tyrphostin blocks the cellular actions of nerve growth factor. Biochemistry. 32:4650–4658. [DOI] [PubMed] [Google Scholar]
- Peters, H.C., H. Hu, O. Pongs, J.F. Storm, and D. Isbrandt. 2005. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci. 8:51–60. [DOI] [PubMed] [Google Scholar]
- Poo, M.M. 2001. Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2:24–31. [DOI] [PubMed] [Google Scholar]
- Raucher, S., and S.E. Dryer. 1995. Target-derived factors regulate the expression of Ca2+- activated K+ currents in developing chick sympathetic neurones. J. Physiol. 486:605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger, J. 2000. Cell signaling by receptor tyrosine kinases. Cell. 103:211–225. [DOI] [PubMed] [Google Scholar]
- Schroeder, B.C., C. Kubisch, V. Stein, and T.J. Jentsch. 1998. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 396:687–690. [DOI] [PubMed] [Google Scholar]
- Schroeder, B.C., M. Hechenberger, F. Weinreich, C. Kubisch, and T.J. Jentsch. 2000. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J. Biol. Chem. 275:24089–24095. [DOI] [PubMed] [Google Scholar]
- Selyanko, A.A., and D.A. Brown. 1996. Intracellular calcium directly inhibits potassium M channels in excised membrane patches from rat sympathetic neurons. Neuron. 16:151–162. [DOI] [PubMed] [Google Scholar]
- Selyanko, A.A., and D.A. Brown. 1999. M-channel gating and simulation. Biophys. J. 77:701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, M.S., J.P. Roche, E.J. Kaftan, H. Cruzblanca, K. Mackie, and B. Hille. 2000. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K+ channels that underlie the neuronal M current. J. Neurosci. 20:1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, M.S., L.P. Wollmuth, and B. Hille. 1994. Angiotensin II inhibits calcium and M current channels in rat sympathetic neurons via G proteins. Neuron. 12:1319–1329. [DOI] [PubMed] [Google Scholar]
- Singh, N.A., C. Charlier, D. Stauffer, B.R. DuPont, R.J. Leach, R. Melis, G.M. Ronen, I. Bjerre, T. Quattlebaum, J.V. Murphy, et al. 1998. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat. Genet. 18:25–29. [DOI] [PubMed] [Google Scholar]
- Skok, V.I., and A.Y. Ivanov. 1983. What is the ongoing activity of sympathetic neurons? J. Auton. Nerv. Syst. 7:263–270. [DOI] [PubMed] [Google Scholar]
- Storm, J.F. 1989. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J. Physiol. 409:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh, B.C., and B. Hille. 2002. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 35:507–520. [DOI] [PubMed] [Google Scholar]
- Suh, B.C., and B. Hille. 2005. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 15:370–378. [DOI] [PubMed] [Google Scholar]
- Tatulian, L., P. Delmas, F.C. Abogadie, and D.A. Brown. 2001. Activation of expressed KCNQ potassium currents and native neuronal M-Type potassium currents by the anti-convulsant drug retigabine. J. Neurosci. 21:5535–5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian, L., and D.A. Brown. 2003. Effect of the KCNQ potassium channel opener retigabine on single KCNQ2/3 channels expressed in CHO cells. J. Physiol. 549:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.S., and D. McKinnon. 1995. Potassium currents in rat prevertebral and paravertebral sympathetic neurones: control of firing properties. J. Physiol. 485:319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.S., Z. Pan, W. Shi, B.S. Brown, R.S. Wymore, I.S. Cohen, J.E. Dixon, and D. McKinnon. 1998. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 282:1890–1893. [DOI] [PubMed] [Google Scholar]
- Weems, W.A., and J.H. Szurszewski. 1978. An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J. Neurophysiol. 41:305–321. [DOI] [PubMed] [Google Scholar]
- Winks, J.S., S. Hughes, A.K. Filippov, L. Tatulian, F.C. Abogadie, D.A. Brown, and S.J. Marsh. 2005. Relationship between membrane phosphatidylinositol-4, 5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J. Neurosci. 25:3400–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S.P., C.H. Yeh, S.L. Sensi, B.J. Gwag, L.M. Canzoniero, Z.S. Farhangrazi, H.S. Ying, M. Tian, L.L. Dugan, and D.W. Choi. et al. 1997. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science. 278:114–117. [DOI] [PubMed] [Google Scholar]
- Zaika, O., L.S. Lara, N. Gamper, D.W. Hilgemann, D.B. Jaffe, and M.S. Shapiro. 2006. Angiotensin II regulates neuronal excitability via phosphatidylinositol 4,5-bisphosphate-dependent modulation of Kv7 (M-type) K+ channels. J. Physiol. 575:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika, O., G.P. Tolstykh, D.B. Jaffe, and M.S. Shapiro. 2007. Inositol riphosphate-mediated Ca2+ signals direct purinergic P2Y receptor regulation of neuronal ion channels. J. Neurosci. 27:8914–8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., L.C. Craciun, T. Mirshahi, T. Rohacs, C.M. Lopes, T. Jin, and D.E. Logothetis. 2003. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 37:963–975. [DOI] [PubMed] [Google Scholar]
- Zhang, Y.H., M.R. Vasko, and G.D. Nicol. 2002. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na+ current and delayed rectifier K+ current in rat sensory neurons. J. Physiol. 544:385–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.H., M.R. Vasko, and G.D. Nicol. 2006. Intracellular sphingosine 1-phosphate mediates the increased excitability produced by nerve growth factor in rat sensory neurons. J. Physiol. 575:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]