Summary
Requirements for CD4 memory differentiation were analyzed using adoptively transferred SMARTA TCR transgenic cells specific for an LCMV epitope. Following LCMV infection, effector and memory differentiation of SMARTA cells mimicked the endogenous CD4 response. In contrast, infection with a recombinant Listeria expressing the LCMV epitope resulted in massive expansion of SMARTA cells but rapid loss of effector function and failure to form memory. Defective memory differentiation was seen only after stimulation of naïve, not memory SMARTA cells, was independent of precursor frequency and associated with a gene expression profile indicative of Bim-mediated apoptosis. Failure to form memory correlated with a lower avidity compared to endogenous responders. In addition, long-lived endogenous CD4+ memory T cells skewed to a higher functional avidity over time. These results support a model in which CD4 memory differentiation and longevity depend on the strength of the TCR signal during the primary response.
Introduction
Following acute infection, antigen-specific T cells expand as much as 50,000-fold, acquire effector function and mediate clearance of the pathogen. Following resolution of the infection, 90-95% of antigen-specific T cells die, leaving behind a long-lived population of memory T cells that provide protection upon re-infection (Williams and Bevan, 2007; Kaech and Wherry, 2007). Memory T cells possess several properties crucial for their function, including higher frequencies, the ability to rapidly re-activate upon antigen stimulation, wide tissue distribution, and the ability to survive and self-renew for long periods in the absence of cognate antigen.
In recent years it has become clear that numerous signals during the primary phase of the immune response can impact the differentiation of functional memory. CD4+ T cells are thought to deliver signals important for the survival and protective function of ensuing CD8+ memory T cells, even though the primary CD8+ T cell response to a pathogen is often unaffected by their absence (Janssen et al., 2003; Shedlock and Shen, 2003; Sun and Bevan, 2003). IL-2 signals have also been shown to play an important role in the differentiation of memory cells capable of secondary expansion (Williams et al., 2006; Bachmann et al., 2007). Expression of IL-7Rα on T cells at the peak of the effector response to acute infection correlates with the ability to survive the contraction phase and progress to memory (Kaech et al., 2003), although IL-7 signals themselves do not appear to be sufficient for this process (Hand et al., 2007; Klonowski et al., 2006; Sun et al., 2006). These findings have illustrated the need to accurately define the signals during the primary response that promote the differentiation of memory cells with their distinctive properties.
The role of TCR signals in the development of memory T cells is not fully understood. In one proposed scenario, repeated encounters with antigen drive the continued expansion and differentiation of T cell populations, with higher levels of TCR stimulation driving the differentiation to memory (Gett et al., 2003; Lanzavecchia and Sallusto, 2002). However, another model proposes that once the initial encounter with antigen has reached a certain activation threshold, responding T cells expand and differentiate independently of further TCR signals (Prlic et al., 2007). In support of this, CD8+ T cells stimulated with antigen for a short period in vivo or in vitro can undergo expansion, gain effector function and differentiate to memory (Kaech and Ahmed, 2001; Mercado et al., 2000; van Stipdonk et al., 2003). In vivo antigenic recognition of as little as 6-12 hours, in the context of an inflammatory response, is sufficient to drive effector and memory CD8+ T cell development (Prlic et al., 2006). Inflammatory signals themselves during viral or bacterial infections also preferentially promote the development of end-stage effectors over memory cells (Joshi et al., 2007; Pearce and Shen, 2007).
Evidence indicates that the requirements for effector and memory differentiation differ for CD4+ and CD8+ T cells. A shortened period of stimulation in vivo does not impact the development of the CD8+ T cell effector response but results in a greatly decreased CD4+ T cell response (Obst et al., 2005; Williams and Bevan, 2004), implying a role for continued antigen stimulation in the development of CD4 responses. Furthermore, in some instances CD4+ memory T cells have shown a gradual decline over time following acute infection in mice, in contrast to the stability of CD8+ memory T cell populations (Homann et al., 2001). This seems to conflict with observations of CD4+ memory T cells in humans, which persist for up to 75 years with a half-life similar to that seen for CD8+ memory T cells (Hammarlund et al., 2003). Understanding the differentiation and maintenance of CD4+ memory T cells following infection remains an important focus of study, particularly as it relates to the development of vaccine strategies for the targeted stimulation of CD4+ T cell responses.
We employed a model of adoptive transfer of TCR transgenic T cells to study CD4 memory differentiation in vivo. SMARTA mice express a TCR transgene with specificity for the immunodominant I-Ab-restricted GP61-80 epitope of lymphocytic choriomeningitis virus (LCMV). Small numbers (103-104) of naïve CD4+ SMARTA T cells (Thy1.1+) transferred into C57BL/6 (B6) hosts expanded dramatically following LCMV infection. They also differentiated into cytokine-producing effector cells and long-lived memory cells similarly to endogenous CD4 responders specific for the same epitope. Following infection with a recombinant Listeria monocytogenes secreting GP61-80 (Lm-gp61), naïve SMARTA cells also expanded dramatically (10,000 to 20,000-fold). Following resolution of the Lm-gp61 infection, however, SMARTA cells rapidly lost cytokine-producing function and failed to differentiate into memory, despite the development of readily detectable memory populations by endogenous responders to the same epitope in the same animal. As early as day 5 post-infection, SMARTA cells were doomed to die and could not be rescued through further stimulation. Only naïve SMARTA cells failed to form memory, as memory SMARTA cells initially generated during LCMV infection and then rechallenged with Lm-gp61 readily expanded, differentiated and formed secondary memory populations. Microarray analysis revealed that the failure to differentiate into memory coincided with increased Bim and decreased Bcl-2 expression at the peak of the response. To assess the role of antigen availability in driving CD4+ T cell differentiation in these two model systems, we measured the functional avidity of endogenous and SMARTA CD4 responders following infection, as defined by the dose of antigen required to elicit a functional response (i.e. IFNγ production). Failure of the SMARTA cells to differentiate into memory correlated with decreased functional avidity as compared to the endogenous Lm-gp61 responders in the same host, suggesting that the limited antigen available during Lm-gp61 infection was insufficient to promote SMARTA memory differentiation. In addition, long-lived CD4+ memory T cell populations (>6 months) were characterized by the emergence of cells with higher functional avidity. These data support a model in which the differentiation of effector and memory CD4+ T cells, as well as the longevity of the ensuing CD4+ memory T cell population, is driven by the strength of antigen stimulation during the primary response.
Results
SMARTA cells mimic the endogenous CD4 response to LCMV
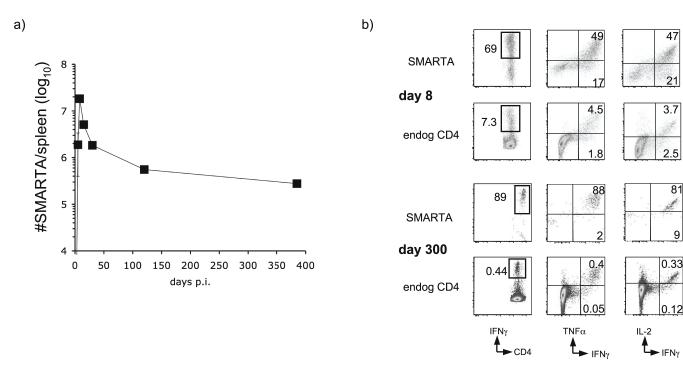
In order to track in vivo CD4+ T cell responses to acute infection, we employed an adoptive transfer system utilizing SMARTA CD4+ TCR transgenic T cells. We transferred 1 × 104 congenically marked (Thy1.1+) CD44lo SMARTA cells specific for the I-Ab restricted GP61-80 epitope of LCMV into B6 hosts one day prior to LCMV infection. SMARTA cells expanded dramatically during the first week of infection in the spleen (Fig. 1a), lymph nodes and peripheral tissues such as the lung and liver (data not shown). By day 30 post-infection, the majority of the SMARTA cells died, leaving behind a long-lived memory population. The memory maintenance phase was characterized by a gradual decline in SMARTA numbers, although substantial numbers of SMARTA cells were readily detectable in the spleen up to 385 days post-infection. The rate of decline in memory cell numbers also decreased over time (Fig. 1A). The overall kinetics of the SMARTA response to LCMV infection largely mirrored the polyclonal endogenous CD4 response to the same GP61-80 epitope (data not shown).
Figure 1.
Adoptively transferred SMARTA cells mimic the endogenous CD4 response to LCMV. a) 1 × 104 CD44lo SMARTA cells (Thy1.1+) were transferred into B6 hosts (Thy1.2+). Mice were infected with LCMV one day later, and the subsequent expansion, contraction and memory maintenance of SMARTA cells in the spleen were calculated based on expression of Thy1.1. Error bars display the SEM (n=3-4 per time point) and results are representative of four different experiments. b) Splenocytes harvested at day 8 or day 300 post-infection were restimulated with GP61-80 peptide for four hours in the presence of Brefeldin A. Cells were then stained for intracellular expression of IFNγ, TNFα and IL-2. We gated on CD4+ Thy1.1+ SMARTA responders and CD4+Thy1.1- endogenous responders from the same animal and assessed their ability to make each cytokine at each time point. Flow plots are representative of effector and memory time points over the course of four time-course experiments.
In addition to displaying similar kinetics, the functional development of the SMARTA cells was similar to that of the endogenous CD4+ T cell responders in the same animal. At both the peak of the effector response (day 8 post-infection) and at memory time points, the bulk of the SMARTA cells maintained the ability to produce IFNγ, IL-2 and TNFα, with a cytokine production profile similar to that of the endogenous CD4+ T cell responders (Fig. 1B). Combined with the kinetics of the SMARTA response, these results led us to conclude that SMARTA cells could be used as an effective surrogate for endogenous CD4+ T cell responses during LCMV infection (Whitmire et al., 2006).
SMARTA cells expand and differentiate following Lm-gp61 infection but fail to form memory
In order to study SMARTA differentiation in another model system, we infected host mice with recombinant Listeria monocytogenes expressing the I-Ab restricted GP61-80 epitope of LCMV under the control of the LLO promoter (Lm-gp61). By day 8 post-infection, SMARTA cells had expanded dramatically, although they were present at lower frequencies and total numbers in the spleen than SMARTA cells 8 days after LCMV infection. By day 60, however, SMARTA cells had completely disappeared from the spleens of Lm-gp61 infected animals (Fig. 2A,D). In contrast, following either infection endogenous CD4+ T cell responders in the same animal specific for the same epitope were readily detectable at the peak of the response (day 8 post-infection) and at memory time points (day 60) (Fig. 2B,D). Similar results were found in the liver (data not shown). A closer look at the day 8 response to Lm-gp61 revealed that while the SMARTA cells expanded several thousand-fold during the first week of infection, they failed to differentiate properly in comparison to the endogenous responders, as measured by their ability to produce cytokines. While the great majority of IFNγ-producing endogenous CD4 responders also produced TNFα and IL-2, only a small proportion of SMARTA cells at this time point retained this ability (Fig. 2C). Furthermore, production of all three cytokines by SMARTA cells in response to peptide stimulation was much lower, as measured by mean fluorescence intensity of intracellular staining.
Figure 2.
SMARTA cells expand but fail to gain full effector function and form memory after Lm-gp61 infection. a) 1 × 104 CD44lo SMARTA cells (Thy1.1+) were transferred into B6 hosts (Thy1.2+). Mice were infected with Lm-gp61 or LCMV one day later, and the expansion, contraction and memory maintenance of SMARTA cells in the spleen were assessed. Representative flow plots display the frequency of Thy1.1+CD4+ SMARTA cells in the spleen at day 8 and day 60 post-infection for each infection. b) Representative flow plots display the frequency of IFNγ-producing endogenous CD4+ responders (CD4+Thy1.1-) in the spleen following peptide restimulation in the presence of Brefeldin A. c) Representative flow plots compare the ability of SMARTA cells (CD4+Thy1.1+) and endogenous CD4+ responders (CD4+Thy1.1-) in the spleen to make IFNγ, TNFα and IL-2 following peptide restimulation at day 8 post-infection. Flow plots are representative of six separate experiments. d) The number of SMARTA (Thy1.1+) and endogenous (IFNγ-producing) responders in the spleen are shown for day 8 and day 60. Error bars are the SEM (n=3/group).
We analyzed the kinetics and cytokine profile of the SMARTA response in greater detail during the first two weeks following Lm-gp61 infection (Fig. 3A). SMARTA cells were readily detectable at five days post-infection. Furthermore, at this early stage their ability to make high levels of both IFNγ and TNFα mirrored that of the endogenous responders. While they continued to expand until day 7 post-infection, most of the SMARTA cells lost the ability to produce TNFα between day 5 and day 7, as compared to the endogenous CD4+ T cell responders that retained the ability to make both cytokines at high levels. We observed a similar decline in the ability of SMARTA cells to produce IL-2 between days 5 and 7 (data not shown). While the number of endogenous responders declined slowly through the contraction phase until day 15 post-infection, SMARTA numbers dropped precipitously. At day 12 they represented only 0.1% of the CD4 population, and by day 15 they were undetectable (Fig. 3A). Furthermore, rechallenge with LCMV at day 5 post-infection with Lm-gp61 failed to rescue the SMARTA cells, as under these conditions SMARTA cells were still undetectable by day 15 post-infection (Supp. Fig. 1). These data indicated that not only were SMARTA cells progressively losing functional capacity by days 5-7 post-infection, they were also irreversibly committed to undergoing programmed cell death.
Figure 3.
Following Lm-gp61 infection, naïve SMARTA cells lose function and disappear while memory SMARTA differentiate normally. a) 1 × 104 CD44lo SMARTA cells (Thy1.1+) were transferred into B6 hosts (Thy1.2+). Mice were infected with Lm-gp61 one day later, and SMARTA cells were subsequently tracked in the spleen at several time points post infection based on Thy1.1 expression (left column). Cytokine production by SMARTA (middle column) and endogenous (right column) CD4 responders from the same animal was assessed at the indicated points. Flow plots are representative of three mice per time point in this experiment, and similar results were found in three separate experiments. b) 1 × 104 CD44lo SMARTA cells (Thy1.1+) were transferred into B6 hosts (Thy1.2+). Mice were infected with LCMV one day later and then rechallenged with Lm-gp61 180 days after the primary infection. Representative flow plots display the frequency of SMARTA cells in the spleen at 7 and 42 days post-rechallenge (top row). The bottom row displays the ability of SMARTA cells at each time point to produce IFNγ and TNFα in response to ex vivo peptide restimulation. Plots are representative of 3-4 mice per group.
We asked whether memory SMARTA cells generated by LCMV infection could form secondary memory following rechallenge with Lm-gp61. Infection with LCMV gave rise to a readily detectable SMARTA memory population 180 days later, with frequencies ranging from 0.2-1.0% of CD4+ T cells in the spleen, lymph nodes and liver (data not shown). Following rechallenge with Lm-gp61 at this time point, SMARTA memory cells expanded dramatically, differentiated normally as measured by IFNγ and TNFα production, and differentiated to readily detectable secondary memory by day 42 post-rechallenge (Fig. 3B). As a control for this experiment, mice receiving naïve SMARTA cells and given a primary challenge with Lm-gp61 failed to develop detectable SMARTA memory cells at day 42 post-infection (data not shown). Therefore, unlike naïve SMARTA cells, memory SMARTA cells, do have the ability to differentiate to secondary memory following Lm-gp61 infection.
Failed SMARTA memory differentiation is independent of precursor frequency
We next considered the possibility that the inability of SMARTA to differentiate into memory following Lm-gp61 infection could depend on the precursor frequency of responding cells. Clonal competition has been shown to play an important role in the selection, differentiation and survival of CD4+ T cell responders in vivo (Foulds and Shen, 2006). We hypothesized that following Lm-gp61 infection competition for limited access to antigen or to differentiation and growth factors due to abnormally high precursor frequencies might prevent normal memory differentiation of the SMARTA cells. By assuming a “take” of ∼10% of adoptively transferred cells, as confirmed with high frequency transfers (data not shown), we estimated that our previous transfers of 1 × 104 SMARTA cells resulted in a precursor frequency of ∼1 × 103, or roughly 5-20-fold higher than the estimated endogenous precursor frequency for this epitope (Whitmire et al., 2006).
To test the possibility that clonal competition prevented SMARTA memory differentiation, we transferred 1 × 103, 1 × 104 or 1 × 105 SMARTA cells into naïve B6 mice that were infected with Lm-gp61 one day later. At day 7 post-infection, SMARTA cells were readily detectable in the spleens of all groups, with the highest frequencies and largest numbers harvested from the group that received 1 × 104 SMARTA cells (Fig. 4A-B). However, the greatest expansion was observed in the group that received 1 × 103 SMARTA cells. Assuming a 10% “take” and estimating an actual precursor frequency of ∼100 SMARTA cells in this group (which is similar to that of endogenous precursor frequencies), we calculated a fold expansion of 12,000-16,000 by SMARTA cells during the first 7 days following Lm-gp61 infection (Fig. 4C). Remarkably, however, such massive expansion was not accompanied by enhanced differentiation, as measured by their ability to make IFNγ, TNFα and IL-2 (data not shown), nor did it confer the properties necessary for memory generation, as SMARTA cells were undetectable in all groups by day 50 post-infection regardless of precursor frequency (Fig. 4A). Therefore, we concluded that the signals required for CD4 memory differentiation can be disassociated from those required for robust clonal expansion during the primary phase of the response. Furthermore, clonal competition could not be invoked to explain the inability of SMARTA cells to form memory following Lm-gp61 infection.
Figure 4.
SMARTA cells fail to differentiate into memory following Lm-gp61 infection regardless of naïve precursor frequency. a) 1 × 103, 1 × 104 or 1 × 105 CD44lo SMARTA cells (Thy1.1+) were transferred into B6 hosts (Thy1.2+) that were infected with Lm-gp61 one day later. Representative flow plots display the frequency of Thy1.1+ SMARTA cells among total CD4+ T cells at days 7 and 50 post-infection for each cell dose. b) Bar graph displays the number of SMARTA cells harvested at day 7 post-infection following transfer of the indicated number of naïve SMARTA cells. c) Bar graph displays the fold expansion of SMARTA cells at each cell dose during the first week post-infection. Starting numbers were calculated based on a 10% “take” of transferred cells. Error bars are the SEM (n=3/group).
SMARTA gene expression profile
In order to explore the mechanism leading to the death of SMARTA responders following Lm-gp61 infection, we analyzed the gene expression profiles of SMARTA cells 7 days following infection with either LCMV or Lm-gp61 using gene expression microarrays (Table I). This analysis demonstrated stark differences in the gene expression profile of SMARTA cells responding to each type of infection. SMARTA cells following Lm-gp61 infection demonstrated increased expression of 65 genes of known function (as defined by an average ≥3-fold increase in expression in two separate experiments). Of these, 45 had known suppressive or regulatory activities as transcriptional repressors (e.g. TSC-22, Tob2), inhibitors of cell cycle or cellular activation (e.g. Rap2A, Unc5cl, PP2A, Ptger4), or pro-apoptotic factors (e.g. Bim, Nor-1, FoxO3a, FasL). In contrast, following LCMV infection over 300 genes demonstrated increased expression, including anti-apoptotic factors (e.g. Bcl-2, Hsp110, SODD), growth and activation factors (e.g. β-catenin, TCF-1, CD27, CD122), trafficking receptors (e.g. CXCR5, CD43), genes involved in metabolic pathways, cell cycle genes, chromatin remodeling genes, transcription factors associated with cellular activation, and various regulatory factors associated with down- modulation of TCR signals and cellular activation. These data indicate that SMARTA cells at day 7 following Lm-gp61 infection are undergoing apoptosis and are refractory to activation signals. Of particular interest is the discoordinated expression of Bim and Bcl-2, two molecules that have been shown to have opposing effects on T cell survival during various phases of the immune response (Marrack and Kappler, 2004). The microarray results for Bcl-2, β-catenin, TCF-1, Nor-1 and Bim have been further confirmed by real time PCR (Supp. Fig. 2). Intracellular staining also revealed decreased levels of Bcl-2 protein expression following Lm-gp61 infection (Supp. Fig. 2).
Table I. Microarray analysis of SMARTA responders 7 days after infection with Lm-gp61 or LCMV.
The columns on the left display genes whose upregulated expression is induced by Lm-gp61, as compared to LCMV, while the right columns display genes upregulated by LCMV
| Increased expression: Lm-gp61 | |||
|---|---|---|---|
| gene product | fold inc. | function | ref. |
| Bim | 12 | apoptosis | (Marrack and Kappler, 2004) |
| Nor-1 | 8 | TCR-induced apoptosis | (Cheng et al., 1997) |
| FoxO3a | 5 | tolerance/apoptosis | (Lin et al., 2004; Stahl et al., 2002) |
| FasL | 7 | apoptosis/AICD | (Green et al., 2003) |
| Unc5cl | 4 | apoptosis (death domain)/inhibits NF-kB | (Zhang et al., 2004) |
| Bach-2 | 9 | transcriptional repressor/apoptosis | (Muto et al., 2002) |
| TSC-22 | 5 | transcriptional repressor of activation, prolif. | (Kester et al., 1999) |
| Ssbp2 | 4 | transcriptional repressor of growth and differentiaiton | (Liang et al., 2005) |
| Zfhx1a | 3 | transcriptional repressor, cell cycle arrest | (Chen et al., 2006) |
| Tis7 | 3 | transcriptional repressor | (Vietor and Huber) |
| Tob2 | 3 | transcriptional repressor of T cell activation | (Shunji Jia, 2007) |
| FoxN3 | 3 | transcriptional repressor of tumorigenic genes | (Scott and Plon, 2005) |
| Rap2A | 7 | Prevents Akt activation | (Christian et al., 2003) |
| PP2A | 5 | Suppresses CTLA4-mediated Akt signals | (Parry et al., 2005) |
| Smad2 | 4 | TGF signalling | (Mamura et al., 2000) |
| March7 | 3 | suppresses T cell activation, proliferation (Ub. ligase) | (Metcalfe et al., 2005) |
| SLAP | 3 | TCR degradation | (Myers et al., 2005) |
| Pdcd4 | 3 | programmed cell death, AP-1 inhibitor | (Bitomsky et al., 2004) |
| Ptger4 | 3 | Suppresses CD4 T cell activation | (Kabashima et al., 2002) |
| Cyclin D2 | 4 | cell cycle | |
| PI3K | 3 | TCR-induced T cell activation | |
| ∼70% of upregulated mRNA with gene products of known function encode genes with described or predicted involvement in suppression of cell proliferation, activation, differentiation or promotion of apoptosis (45/65). | |||
| Increased expression: LCMV | |||
|---|---|---|---|
| gene product | fold inc. | function | ref. |
| Bcl-2 | 8 | anti-apoptotic | (Marrack and Kappler, 2004) |
| SODD | 4 | Silencing of death domains, anti-apoptotic | (Jiang et al., 1999) |
| Hsp110 | 11 | anti-apoptotic, stress response | (Yamagishi et al., 2006) |
| β-catenin | 9 | T cell survival, proliferation | (Qiang and Rudikoff, 2004) |
| TCF-1 | 8 | T cell survival, proliferation | (Qiang and Rudikoff, 2004) |
| CD27 | 5 | T cell survival, differentiation | (Hendriks et al., 2003) |
| CD122 | 3 | T cell growth, survival | (Waldmann, 2006) |
| CD43 | 9 | T cell expansion, migration | (Onami et al., 2002) |
| CXCR5 | 9 | T cell trafficking to germinal center | (Breitfeld et al., 2000; Schaerli et al., 2000) |
| >300 genes of known function demonstrated upregulated expression (data not shown) and can generally be segregated into the following categories: • Stress response/DNA repair • Metabolic pathways • Cell cycle • Signaling pathways of cellular activation, proliferation and survival • Chromatin structure • Trafficking receptors (e.g. CXCR5) • Growth/survival factor receptors (e.g. CD122) • Negative regulators of TCR-mediated signaling • Transcription factors associated with cellular activation | |||
Together, these data strongly suggest that stimulation of the SMARTA cells during Lm-gp61 infection induces rapid expansion but defective differentiation and eventual programmed cell death. Given the rapid disappearance of the SMARTA cells, one concern was that they are rejected during Listeria infection, whether due to the expression of the Thy1.1 congenic marker or minor histocompatibility differences not eliminated during backcrossing. Several lines of evidence indicate that this is not the case. First, the loss of function as reflected by cytokine production is indicative of failed differentiation, not rejection. Second, the gene expression profile is also indicative of failed differentiation and programmed cell death, with increased expression of key apoptotic factors. Third, memory SMARTA challenged with Lm-gp61 readily form secondary memory, indicating that the failure of naïve SMARTA to form primary memory following stimulation is likely due to differences in activation threshold, not rejection. To definitively address the issue of rejection, we transferred day 8 SMARTA from either Lm-gp61 or LCMV infected hosts into secondary hosts that were also day 8 post-infection with either Lm-gp61 or LCMV and that had also received an initial SMARTA transfer prior to infection (to ensure that the mice were “primed”). In order to distinguish the secondary SMARTA transfer from the initial SMARTA transfer in these hosts, the transferred SMARTA cells were labeled with CFSE. Seven days later (15 days p.i.), the survival of SMARTA cells in the spleen was assessed. Although some CFSE dilution was observed, primary and secondary SMARTA transfers were distinguishable. As expected, SMARTA cells generated in a Lm-gp61 infected host and injected into a Lm-gp61 infected host largely disappeared, while SMARTA cells generated in a LCMV-infected host and transferred into a LCMV-infected host were readily detectable. Conversely, SMARTA cells generated in a Lm-gp61 infected host and injected into a LCMV infected host disappeared within seven days, while SMARTA cells generated in a LCMV-infected host and transferred into a Lm-gp61-infected host demonstrated no impairment in survival (Supp. Fig. 3). We were therefore unable to detect any mechanism for rejecting SMARTA cells in Lm-gp61-infected animals and definitively ruled out this possible explanation for their disappearance.
Failed SMARTA memory differentiation correlates with low functional avidity
Previous studies have indicated that CD4+ T cells undergo avidity maturation throughout the primary response to pathogen as well as during subsequent rechallenges (Savage et al., 1999; Whitmire et al., 2006). These results support the idea that CD4+ T cell effector differentiation and the selection of responding repertoires is driven at least in part by the strength of antigenic stimulation. Furthermore, the observation that memory SMARTA challenged with Lm-gp61 readily differentiated into secondary memory suggested the possibility that their ability to differentiate could be due to the lower activation threshold of memory cells. Therefore, we hypothesized that the levels of antigen available on the APC could also impact the selection of CD4+ T cell responders for differentiation into memory. In this scenario, a certain level of TCR stimulation might be required for clonal expansion, but further stimulation would be required for memory differentiation. In our model system, high levels of antigen density provided by LCMV infection might promote the expansion and differentiation of CD4 responders over a wider range of functional avidities, while lower levels of antigen density provided by Lm-gp61 infection might allow memory differentiation of only high functional avidity responders. SMARTA cells, if they were of low avidity, would be able to initially expand and differentiate after Lm-gp61 infection but would lack sufficient TCR signals to progress to memory.
We tested this hypothesis by measuring the functional avidities of SMARTA and endogenous CD4 responders at the peak of the primary response to either LCMV or Lm-gp61. The functional avidity of the responding populations was measured by assessing their ability to produce IFNγ ex vivo in response to decreasing concentrations of their cognate peptide, GP61-80. The maximal I-Ab/GP61-80-restricted response was detected using 10-5 M peptide. With decreasing peptide concentrations, the number of endogenous CD4+ T cell responders after LCMV infection began to decrease immediately, showing a half-maximal response between 1 × 10-7 M and 3 × 10-7 M peptide. In contrast, virtually all endogenous responders generated after Lm-gp61 infection were able to respond at peptide concentrations as low as 3 × 10-7 M, and they displayed a half-maximal response at ∼5-10-fold lower concentrations of peptide (Fig. 5A-B). While LCMV induced effectors that required high concentrations of peptide for their functional activity (1 × 10-7 M to 1 × 10-5 M), this population was absent in the Lm-gp61-infected animals (Fig. 5B). These results indicated that LCMV infection was able to recruit both intermediate and high functional avidity responders, while Lm-gp61 infection resulted in the effector differentiation of only high avidity cells, supporting the idea that higher levels of antigen are available during LCMV infection.
Figure 5.
SMARTA responders demonstrate lower functional avidity, as compared to endogenous CD4 responders to Lm-gp61 in the same host. a) B6 mice were infected with either LCMV or Lm-gp61. Eight days later splenocytes were restimulated over a range of GP61-80 peptide concentrations and stained for expression of IFNγ and TNFα. Representative flow plots are gated on CD4+ T cells and show the frequency of cytokine producers following restimulation at the indicate peptide concentrations. b) The graph displays the number of endogenous CD4+ T cells in the spleen capable of producing IFNγ in response to decreasing concentrations of peptide. c) 1 × 104 CD44lo SMARTA cells (Thy1.1+) were transferred into B6 hosts (Thy1.2+), and mice were infected with LCMV one day later. The graph displays the percent maximal endogenous or SMARTA CD4 response induced by ex vivo peptide restimulation, as measured by the frequency of IFNγ-producing responders at each peptide concentration divided by the frequency of IFNγ-producing responders at the highest peptide concentration (1 × 10-5 M). d) 1 × 104 CD44lo SMARTA cells (Thy1.1+) were transferred into B6 hosts (Thy1.2+), and mice were infected with Lm-gp61 one day later. The graph displays the percent maximal endogenous or SMARTA CD4 response induced by ex vivo peptide restimulation, as measured by the frequency of IFNγ-producing responders at each peptide concentration divided by the frequency of IFNγ-producing responders at the highest peptide concentration (1 × 10-5 M). The error bars are the SEM (n=3 for each group). Results are representative of four separate experiments. e) 1 × 103 CD44lo SMARTA cells (Thy1.1+) were transferred into B6 hosts (Thy1.2+), and mice were infected with LCMV or Lm-gp61 one day later. Decreasing concentrations of gp66-77/I-Ab Class II tetramer was used to stain SMARTA and endogenous responders at day 8 post-infection. The graph displays the number of tetramer-positive endogenous cells detected at each concentration, normalized to staining with the control tetramer hCLIP/I- Ab. f) and g) The graph displays the percentage of tetramer-positive cells as compared to the number of tetramer-positive cells at the highest tetramer concentration following LCMV or Lm-gp61 infection. Error bars are the SEM (n=4/group).
We then asked where SMARTA responders fell in the functional avidity spectrum. SMARTA effector cells generated following LCMV infection demonstrated a similar range of functional avidities as compared to the endogenous responders in the same host (Fig. 5C). SMARTA effector cells generated following Lm-gp61 infection, in contrast, displayed decreased functional avidity as compared to the endogenous Lm-gp61 responders to the same epitope in the same host (half-maximal responses induced by peptide concentrations of ∼2 ×10-7 M for SMARTA responders and ∼5 × 10-8 M for endogenous CD4 responders)(Fig. 5D).
Since it is possible that differences in functional avidity may not reflect differences in actual TCR avidity, we also assessed the ability of SMARTA and endogenous responders to bind Class II tetramers for this epitope (gp66-77/I-Ab). We found that tetramer staining of both LCMV and Lm-gp61 responders was similar in frequency to the number of responders detected via intracellular cytokine staining (Supp. Fig. 4). We then tested the ability of both SMARTA and endogenous responders to bind decreasing concentrations of tetramer in an equilibrium binding assay. All staining was normalized to the negative control tetramer, hCLIP/I-Ab. As with the functional avidity assays, we found that following LCMV infection the number of tetramer-binding cells began to decrease as soon as the tetramer was diluted below 8 μg/ml. In contrast, the number of Lm-gp61 responders binding tetramer remained stable at 4-fold lower concentrations, then began to decline as tetramer was diluted further (Fig. 5E). We compared the ability of SMARTA and endogenous responders to bind tetramer by calculating the number of tetramer-positive cells at each concentration as a percentage of tetramer-positive cells at the highest concentration. These results again corresponded to those obtained with the functional avidity assay. The ability of SMARTA cells to bind tetramer in LCMV-infected hosts mirrored that of the endogenous responders (Fig. 5F), whereas the ability of SMARTA cells to bind tetramer in Lm-gp61 infected hosts was lower than that of the endogenous responders (Fig. 5G). We plotted our results using Scatchard analysis and calculated apparent KD as previously described (Savage et al. 1999). This revealed that while the apparent KD of SMARTA cells following LCMV infections was similar to endogenous responders in the same host (17.1 nM vs. 16.6 nM), the KD of endogenous responders to Lm-gp61 was significantly lower (3.2 nM) than either SMARTA responders in the same host (18.3 nM, p=.01) or endogenous responders to LCMV (16.6 nM, p=.02)(Supp. Fig. 5). Our results indicate that the inability of SMARTA cells to form memory corresponds to a decreased ability to compete for TCR signals. These findings support the idea that decreased availability of antigen during Lm-gp61 infection is sufficient to drive expansion and partial effector differentiation of SMARTA cells but is not sufficient for their differentiation into memory.
One possible explanation for these results is that responders to each pathogen are recognizing slightly different determinants of the gp61-80 epitope. Because the tetramer only incorporates the gp66-77 12-mer, we find this explanation unlikely. The frequency of tetramer-binding cells in each infection corresponded to the number of IFNγ-producing cells following gp61-80 peptide restimulation. Furthermore, the avidity, as measured with the tetramer, corresponded to the functional avidity, as measured with the 20-mer gp61-80 (Fig. 5). These results indicate that the repertoire of responding cells in each type of infection is likely responding to the same epitope. As a preliminary analysis or TCR repertoires, we analyzed Vβ usage by tetramer-positive cells (Supp. Fig. 6). We found two staining patterns: subsets that stained an equal number of LCMV and LM-gp61 responders (Vβ2,3,5,6,13) and subsets that stained more LCMV responders than Lm-gp61 responders (Vβ4,7,8.1/8.2,8.3,14). We did not observe any unique Vβ subset usage by Lm-gp61 responders. These findings are consistent with the idea the Lm-gp61 responding repertoire is a subset of the LCMV responding repertoire.
Functional avidity maturation of long-lived CD4 memory
By analyzing the functional avidities of the endogenous CD4+ T cell memory populations following either LCMV or Lm-gp61 infection, we further explored the role of signal strength during primary infection in promoting memory differentiation and longevity. We noted that endogenous responders with higher functional avidity were more likely to transition from effector cells to memory cells (Fig. 6A-B). Even a monoclonal population of SMARTA cells displayed a skewing toward higher functional avidity in the transition to memory following LCMV infection (Fig. 6C), agreeing with studies indicating that responding T cells can distribute over a range of functional avidities regardless of TCR affinity (Kroger and Alexander-Miller, 2007; Slifka and Whitton, 2001). Furthermore, endogenous memory cells generated by either LCMV or Lm-gp61 infection displayed a continuous skewing over time towards higher functional avidity (Fig. 6D-E). While the overall population of memory cells decreases in number over time, those memory cells with higher functional avidity survive preferentially. To illustrate this, if only the numbers of responders with high functional avidity (i.e. those that respond to peptide concentrations ≤1 × 10-8 M) are plotted over time, they display virtually no decay during the memory maintenance phase (Supp. Fig. 7). These findings suggest that the strength of the stimulatory signal delivered to CD4+ T cells during the primary response can dictate not only recruitment into the response, but also memory differentiation and the subsequent longevity of memory cell populations.
Figure 6.
CD4+ memory T cells skew to higher functional avidity over time. a) B6 mice were infected with LCMV. At 8 or 30 days post-infection, splenocytes were restimulated over a range of GP61-80 peptide concentrations ex vivo and stained for expression of IFNγ and TNFα. The graph displays the percent maximal endogenous CD4 response induced by ex vivo peptide restimulation, as measured by the frequency of cytokine-producing responders at each peptide concentration divided by the frequency of cytokine-producing responders at the highest peptide concentration (1 × 10-5 M). b) Similar experiments were performed following Lm-gp61 infection. c) Similar experiments were performed as in a, except that 1 × 104 CD44lo SMARTA cells were transferred one day prior to LCMV infection and the functional avidity of Thy1.1+ SMARTA cells was assessed. d) and e) Experiments similar to those in a and b were performed, except that functional avidity was assessed at days 60 and 180 post-infection.
Discussion
The TCR genes to create the SMARTA transgenic originated from an LCMV-immunized mouse (Oxenius et al., 1998). Our data, plus that of others, show that SMARTA cells serve as an ideal model to follow the in vivo response to LCMV infection through the expansion, contraction and memory phases (Whitmire et al., 2006). Following infection with recombinant Listeria expressing the same epitope however the situation is much different. We found to our surprise that even though the SMARTA expansion phase was intact, the effector population rapidly contracted to zero and left no memory cells. In mice in which the frequency of SMARTA cells approached the endogenous precursor frequency for the gp61 epitope, SMARTA cells expanded 12,000-20,000-fold, representing at least 12-14 cell divisions, but by around day 15 post-infection 100% of the cells had died. Meanwhile, in the same mice, there was an apparently normal expansion, contraction and memory response by the endogenous CD4+ T cell population.
Up until day 5 post-infection with Lm-gp61, effector differentiation of expanding SMARTA cells, as measured by the ability to produce IFNγ, TNFα and IL-2, mirrored that of endogenous CD4 responders to the same epitope. After this point, the cytokine production profile of the SMARTA cells was impaired. Even day 5 SMARTA cells in Lm-gp61-infected mice were refractory to further stimulation, as tested by challenge with LCMV, and were already slated for death. In contrast, endogenous CD4 responders were boosted by the day 5 LCMV rechallenge, agreeing with a prior study indicating that effector T cells at the peak of the primary response retain proliferative potential (Wong et al., 2004). These results indicate that sufficient signaling for clonal expansion and at least partial differentiation of effector function can occur without receiving the signals required for memory differentiation.
We propose that increasing levels of antigenic stimulation promote hierarchical stages of differentiation. In this model, increasing stimulation would first promote clonal expansion and subsequently effector differentiation, differentiation of short-lived memory cells, and finally differentiation of long-lived memory cells. The model is supported by our finding that the inability of SMARTA cells to differentiate into memory following Lm-gp61 infection, despite their massive clonal expansion and initial development of effector function, correlates with a lower range of functional avidity demonstrated by the SMARTA cells compared to the endogenous CD4 responders to the same epitope. We propose that the inability of SMARTA cells to receive proper signals for memory differentiation in this system is largely driven by antigen availability on the APC. The avidity of responding T cell populations has been shown to inversely correlate with antigen dose (Rees et al., 1999). We observed that endogenous responders to the GP61-80 epitope following Lm-gp61 infection were of high functional avidity, whereas endogenous responders to the same epitope following LCMV infection were distributed over a range of intermediate to high functional avidity, indicating that antigen availability during LCMV infection was greater and consequently able to recruit lower avidity responders. These results were further confirmed using MHC Class II tetramer binding as a direct measure of TCR avidity. The observation that memory SMARTA rechallenged with Lm-gp61 differentiate normally and form secondary memory fits with the notion that memory cells have a lower activation threshold and can be more efficiently recruited by lower levels of antigen. An alternative explanation would be that once memory differentiation has been programmed during the primary response, the antigen stimulation requirements for differentiation to secondary memory are less stringent.
Several explanations for decreased antigen availability may be proposed, including decreased antigen display on APCs, clonal competition and competiton with other immunodominant epitopes. Of these, we have ruled out clonal competition as a mechanism for regulating antigen stimulation levels in this particular system, although it has been recently reported that in another scenario clonal competition inhibited CD4 memory differentiation (Blair and Lefrancois, 2007). In our studies, even when the frequency of SMARTA cells approached endogenous precursor frequencies memory differentiation was impaired following Lm-gp61 infection. We suggest that the inability of SMARTA cells to differentiate into memory following Lm-gp61 infection does not necessarily represent an abnormal differentiation event. Rather, during Lm-gp61 infection SMARTA cells are representative of clones that normally expand following activation but do not receive sufficient TCR signals for memory differentiation. Conversely, following LCMV infection, where antigen is more abundant and T cell clones with lower avidity TCRs are recruited into the response, SMARTA cells are representative of clones that have received sufficient TCR signals for memory differentiation. SMARTA cells thus provide a snapshot of one differentiation stage during Lm-gp61 infection and highlight the heterogeneous nature of the effector CD4 population.
We definitively show that the disappearance of SMARTA cells following Lm-gp61 infection is not due to some unforeseen rejection artifact peculiar to Lm-gp61 infection but represents a genuine differentiation defect. In all, these results provide a comprehensive picture of CD4 differentiation during an immune response in which the signaling threshold for clonal expansion is met prior to those for effector differentiation and development of short-lived and long-lived memory populations. Antigen-driven signals do not represent the whole story, however, as even among the CD4 responders of the highest functional avidity significant contraction is observed, indicating that other signals are required for the differentiation of CD4 memory T cells. Also, it is unclear to what extent these findings are influenced by the distinct inflammatory environments of each infection model, as differences in the inflammatory milieu may have substantial effects on T cell differentiation. Recent studies have found that signaling by inflammatory mediators such as IL-12 or Type I IFNs can preferentially promote the effector differentiation of CD8 T cells and prevent their differentiation down a memory pathway, possibly through induction of high levels of the transcription factor T-bet (Pearce and Shen, 2007; Joshi et al., 2007). It is unclear to what extent these findings apply to CD4 memory differentiation. The above studies do not propose that the inflammatory environment represents an all-or-nothing switch away from memory differentiation, which is what we find in our model. We observe normal differentiation of endogenous responders and of memory SMARTA rechallenged with Lm-gp61, arguing against the inflammatory environment being the determining factor for memory differentiation in our system. Furthermore, we observe defective effector differentiation at the peak of the response, indicating that the defect in SMARTA cells goes beyond a switch from a memory differentiation pathway to an effector differentiation pathway. Future studies are needed to assess the impact of modulating antigen presentation without changing the inflammatory environment and other factors such as infectious load, duration of antigen presentation and the nature of the APC.
Notably, Bim expression was increased in SMARTA effector cells following Lm-gp61 infection. Bim has been shown to be required during the contraction phase of the immune response (Pellegrini et al., 2003; Wojciechowski et al., 2006), and may influence other aspects of naïve and memory T cell homeostasis (Wojciechowski et al., 2007). One intriguing possibility is that the extent of antigen stimulation may influence Bim expression in CD4+ T cells. The role of Bim in this system is unclear, however. While Bim upregulation may explain the disappearance of the SMARTA cells after Lm-gp61 infection, it does not explain the loss of cytokine-producing capability nor the inability of SMARTA cells to respond to further antigenic signals within 5 days post-infection, especially since Bim is not differentially expressed in SMARTA cells until day 7 post-infection (data not shown). It remains possible that Bim is a crucial mediator of T cell death caused by insufficient TCR-mediated differentiation signals, but it is also possible that Bim is only one byproduct of a differentiation program gone awry.
Several years ago Lanzavecchia’s group described the progressive differentiation model of the T cell response, in which successive antigen signals drive the progressive clonal expansion and differentiation of responding T cells (Lanzavecchia and Sallusto, 2002). Subsequent in vitro studies have shown that T cell fitness and survival are dependent on the initial strength of the antigenic stimulus (Gett et al., 2003). More recent studies of the CD8 T cell response have instead suggested a model of programmed differentiation, in which a short encounter with antigen promotes the antigen-independent expansion and differentiation of responding T cells (Prlic et al., 2007; Kaech and Wherry, 2007). CD4+ T cells, however, appear to require a more extended period of antigen stimulation for the induction of a robust response (Obst et al., 2005; Williams and Bevan, 2004). This is supported by the observation that CD4+ T cell responders undergo functional avidity maturation throughout the primary response (Whitmire et al., 2006). Also, the progressive skewing towards a high avidity repertoire throughout both primary and secondary CD4 responses suggests an extended period of antigen-driven repertoire selection (Savage et al., 1999). Overall, the available evidence indicates that the forces regulating the induction of CD4 responses are distinct from and more tightly regulated by antigen exposure than those guiding CD8 responses.
It has recently been shown that in some mouse models CD4+ memory T cell populations do not share the stability of CD8+ memory T cell populations, declining slowly over time (Homann et al., 2001). In our studies of CD4 memory, we have also observed a steady, if slow, decline in CD4 memory populations, both by endogenous and SMARTA memory cells. However, we made two pertinent observations regarding this decline. First, the rate of decline slowed over time, both for SMARTA and endogenous CD4 responders. In fact, we were unable to detect a significant decline in endogenous memory cells beyond six months post-infection. Second, surviving memory cell populations consistently skewed to an ever-higher functional avidity over time. This may represent the outgrowth of clones with high functional avidity or the functional maturation of the memory population as a whole. Secondary challenge of mice has been shown to select for responding clones with high avidity (Rees et al., 1999), suggesting that the skewing we observe is likely attributable to the preferential survival of memory cells with high functional avidity. These data suggest that not only does TCR signal strength during the primary response impact the generation of CD4 memory, but also influences the longevity CD4 memory populations. We propose that long-lived CD4 memory cells represent those responders with high functional avidity that received the strongest stimulus during the primary response. This also differs from implications for CD8 memory cells where it is suggested that excessive stimulation, either from antigen or inflammatory mediators, drives the differentiation of end-stage effector CTL that have lost the capacity for memory differentiation (Joshi et al., 2007). In these settings, it is likely that increasing levels of stimulation decrease CD8 memory differentiation potential, the opposite of the scenario we envisage for CD4+ T cells.
It is unclear what the long-term consequences of narrowing of the CD4 repertoire during memory commitment and maintenance might be. While T cells with high functional avidity might be presumed to generate the most potent recall response and be able to detect extremely low levels of antigen, repertoire diversity also has a place in protection from pathogens. Further studies should elucidate the precise role of antigen-driven stimulation in memory development, as well as the consequences of an emerging, narrower repertoire of high-avidity CD4+ memory T cells.
Experimental Procedures
Mice and infections
6-8 week C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME). SMARTA TCR transgenic mice (Oxenius et al., 1998) were maintained in SPF facilities at the University of Washington and the University of Utah. All animal experiments were conducted with the approval of the corresponding IACUC committees at each institution. LCMV Armstrong 53b was grown in BHK cells and titered in Vero cells as described (Ahmed et al., 1984). Mice were infected intraperitoneally (i.p.) with 2 × 105 plaque-forming units (PFU). Lm-gp61 expressing the gp61-80 epitope of LCMV (a gift from M. Kaja-Krishna, University of Washington, Seattle, WA)(Way et al., 2007) was constructed using described methods (Shen et al., 1995) and propagated in BHI broth and on agar plates. Prior to infection, the bacteria were grown to log phase and concentration determined by measuring the O.D. at 600 nm (O.D. of 1 = 1 × 109 CFU/ml). Mice were injected intravenously (i.v.) with 2 × 105 colony forming units (CFU).
Adoptive transfers
Splenocyte cell suspensions were generated from SMARTA mice. Untouched CD4+ T cells were isolated by incubation with a biotinylated antibody cocktail followed by anti-biotin magnetic beads and depletion on a magnetic column, per manufacturer’s recommendations (Miltenyi). In addition, we added biotinylated CD44 antibody (eBiosciences, San Diego, CA) to deplete CD44hi “memory phenotype” SMARTA. TCR transgenic T cell purity was assessed by staining with CD44, Vα2 and Vβ8.3 antibodies, followed by flow cytometric analysis. SMARTA cells were resuspended in PBS and injected i.v. into recipient mice one day prior to infection. In some experiments, SMARTA cells were incubated with 5 mM 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) (Invitrogen) for 10 minutes in warm RPMI.
Microarray and RT-PCR
RNA was isolated using Trizol (Invitrogen) from FACS-sorted SMARTA cells (day 8 post-infection). Message was amplified and converted to biotinylated, fragmented cRNA using a commercially available kit per manufacturer’s instructions (Ambion, Austin, TX). Two biological duplicates from each group were hybridized to Affymetrix Mouse 430 2.0 arrays. Results were normalized (GC-RMA, Bioconducter) and analyzed for differences in log2 expression values. Target genes were identified for further study based on >2.5-fold increased expression in two separate experiments, with a minimum mean of ≥3-fold increased expression. Microarray data has been submitted to the on-line depository GEO (accession # GSE10094) and conforms to all MIAME guidelines. For RT-PCR, first-strand cDNA synthesis and SYBR-green based analysis of real-time PCR amplification was performed using a commercially available kit (Invitrogen) and a Lightcycler 480 (Roche). The following exon-spanning primer pairs were used (5′→3′): Bim: forward-CGGATCGGAGACGAGTTCA, reverse-TTCAGCCTCGCGGTAATCA-; Nor-1: forward-GATCACAGAGCGACATGGGTTA, reverse-GAGCCTGTCCCTTCCTCTGG; Bcl-2: forward- GTGGTGGAGGAACTCTTCAGGGATG, reverse- GGTCTTCAGAGACAGCCAGGAGAAATC; TCF-1: forward- AGTCCCACAGTGTCCTCCAG, reverse- CACGGTTACTGGGAAGAGGA; β-catenin: forward- CCCTGAGACGCTAGATGAGG; reverse- CATGATGGCATGTCTGGAAG. Expression was normalized to GAPDH or HPRT and displayed as a relative fold increase.
Ex vivo restimulation and intracellular cytokine staining
Splenocyte cell suspensions in RPMI supplemented with 10% fetal bovine serum were plated in round-bottom 96-well plates (2-3 × 106 cells/well) and restimulated for 4 hours with 1 μM (or titrated dilutions as indicated in the pertinent figures) GP61-80 peptide from LCMV (GLKGPDIYKGVYQFKSVEFD) in the presence of Brefeldin A (GolgiPlug, 1 μl/ml), per manufacturers instructions (BD Biosciences, San Diego, CA). Following restimulation cells were stained with fluorescently-labeled cell surface antibodies to CD4 and Thy1.1, permeabilized, and stained with fluorescently-labeled antibodies to IFNγ, TNFα and IL-2, using a kit per manufacturers instructions (BD Biosciences, San Diego, CA). Samples were then analyzed by flow cytometry.
Tetramer staining and analysis
The gp66-77/I-Ab tetramer was provided by the NIH Tetramer Core Facility (Emory Vaccine Center, Atlanta, GA). Staining was performed at 37°C for 3 hours in RPMI containing 2% FCS, followed by washing and cell surface staining for CD4, CD44 and Thy1.1. Tetramer fluorescence was normalized to samples stained with control tetramer, hCLIP/I-Ab. Scatchard plots and apparent KD were calculated as described (Savage et al., 1999). Fluorescence units (bound) were plotted on the X-axis, and fluorescence units divided by tetramer concentration (bound/free) were plotted on the Y-axis. KD was determined as the inverse of the slope.
Antibodies and flow cytometry
Cell surface stains were done in PBS containing 1% FBS. Intracellular stains for cytokines and Bcl-2 were done using a kit per manufacturer’s instructions (BD Biosciences). Antibodies conjugated to fluorescent labels were purchased from eBiosciences (San Diego, CA) and include CD4, Thy1.1, IFNγ, IL-2, TNFα, CD62L, and CD44. Antibodies purchased from BD Biosciences (Mountain View, CA) include Bcl-2-PE, Vα2-PE, Vβ8.3-FITC and the Vβ panel staining kit. Flow cytometry was performed using either a FACSCalibur or FACSCanto, and high speed cell sorting was performed using either a FACSVantage or FACSAria (BD Biosciences).
Supplementary Material
Acknowledgements
The authors wish to acknowledge B. Dere, P. Xiao and J. Strickland for technical assistance. We also gratefully acknowledge the NIH Tetramer Core Facility (Atlanta, GA) for providing MHC Class II tetramers. This research was supported by the NIH (M.A.W) and the Howard Hughes Medical Institute (M.J.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur. J. Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- Bitomsky N, Bohm M, Klempnauer KH. Transformation suppressor protein Pdcd4 interferes with JNK-mediated phosphorylation of c-Jun and recruitment of the coactivator p300 by c-Jun. Oncogene. 2004;23:7484–7493. doi: 10.1038/sj.onc.1208064. [DOI] [PubMed] [Google Scholar]
- Blair DA, Lefrancois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proceedings of the National Academy of Sciences. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B Helper T Cells Express CXC Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production. J. Exp. Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yusuf I, Andersen H-M, Fruman DA. FOXO Transcription Factors Cooperate with {delta}EF1 to Activate Growth Suppressive Genes in B Lymphocytes. J Immunol. 2006;176:2711–2721. doi: 10.4049/jimmunol.176.5.2711. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Chan FK, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. Embo J. 1997;16:1865–1875. doi: 10.1093/emboj/16.8.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian SL, Lee RL, McLeod SJ, Burgess AE, Li AHY, Dang-Lawson M, Lin KBL, Gold MR. Activation of the Rap GTPases in B Lymphocytes Modulates B Cell Antigen Receptor-induced Activation of Akt but Has No Effect on MAPK Activation. J. Biol. Chem. 2003;278:41756–41767. doi: 10.1074/jbc.M303180200. [DOI] [PubMed] [Google Scholar]
- Foulds KE, Shen H. Clonal Competition Inhibits the Proliferation and Differentiation of Adoptively Transferred TCR Transgenic CD4 T Cells in Response to Infection. J Immunol. 2006;176:3037–3043. doi: 10.4049/jimmunol.176.5.3037. [DOI] [PubMed] [Google Scholar]
- Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- Green DR, Droin N, Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor {alpha} is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proceedings of the National Academy of Sciences. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks J, Xiao Y, Borst J. CD27 Promotes Survival of Activated T Cells and Complements CD28 in Generation and Establishment of the Effector T Cell Pool. J. Exp. Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MBA. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of Constitutive TNF Receptor 1 Signaling by Silencer of Death Domains. Science. 1999;283:543–546. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation Directs Memory Precursor and Short-Lived Effector CD8+ T Cell Fates via the Graded Expression of T-bet Transcription Factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, Tsuboi K, Sugimoto Y, Kobayashi T, Miyachi Y, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Invest. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and Cell-Fate Decisions in Effector and Memory CD8+ T Cell Differentiation during Viral Infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kester HA, Blanchetot C, den Hertog J, van der Saag PT, van der Burg B. Transforming Growth Factor-beta -stimulated Clone-22 Is a Member of a Family of Leucine Zipper Proteins That Can Homo- and Heterodimerize and Has Transcriptional Repressor Activity. J. Biol. Chem. 1999;274:27439–27447. doi: 10.1074/jbc.274.39.27439. [DOI] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. Cutting Edge: IL-7-Independent Regulation of IL-7 Receptor {alpha} Expression and Memory CD8 T Cell Development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger CJ, Alexander-Miller MA. Cutting Edge: CD8+ T Cell Clones Possess the Potential to Differentiate into both High- and Low-Avidity Effector Cells. J Immunol. 2007;179:748–751. doi: 10.4049/jimmunol.179.2.748. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Liang H, Samanta S, Nagarajan L. SSBP2, a candidate tumor suppressor gene, induces growth arrest and differentiation of myeloid leukemia cells. 2005;24:2625–2634. doi: 10.1038/sj.onc.1208167. [DOI] [PubMed] [Google Scholar]
- Lin L, Hron JD, Peng SL. Regulation of NF-[kappa]B, Th Activation, and Autoinflammation by the Forkhead Transcription Factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Mamura M, Nakao A, Goto D, Kato M, Saito Y, Iwamoto I. Ligation of the T Cell Receptor Complex Results in Phosphorylation of Smad2 in T Lymphocytes. Biochemical and Biophysical Research Communications. 2000;268:124–127. doi: 10.1006/bbrc.2000.2086. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J. Control of T Cell Viability. Annual Review of Immunology. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early Programming of T Cell Populations Responding to Bacterial Infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- Metcalfe SM, Muthukumarana PADS, Thompson HL, Haendel MA, Lyons GE. Leukaemia inhibitory factor (LIF) is functionally linked to axotrophin and both LIF and axotrophin are linked to regulatory immune tolerance. FEBS Letters. 2005;579:609–614. doi: 10.1016/j.febslet.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Muto A, Tashiro S, Tsuchiya H, Kume A, Kanno M, Ito E, Yamamoto M, Igarashi K. Activation of Maf/AP-1 Repressor Bach2 by Oxidative Stress Promotes Apoptosis and Its Interaction with Promyelocytic Leukemia Nuclear Bodies. J. Biol. Chem. 2002;277:20724–20733. doi: 10.1074/jbc.M112003200. [DOI] [PubMed] [Google Scholar]
- Myers MD, Dragone LL, Weiss A. Src-like adaptor protein down-regulates T cell receptor (TCR)-CD3 expression by targeting TCR{zeta} for degradation. J. Cell Biol. 2005;170:285–294. doi: 10.1083/jcb.200501164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obst R, van Santen H-M, Mathis D, Benoist C. Antigen persistence is required throughout the expansion phase of a CD4+ T cell response. J. Exp. Med. 2005;201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onami TM, Harrington LE, Williams MA, Galvan M, Larsen CP, Pearson TC, Manjunath N, Baum LG, Pearce BD, Ahmed R. Dynamic Regulation of T Cell Immunity by CD43. J Immunol. 2002;168:6022–6031. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Shen H. Generation of CD8 T Cell Memory Is Regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proceedings of the National Academy of Sciences. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J. Exp. Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Current Opinion in Immunology. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Qiang YW, Rudikoff S. Wnt signaling in B and T lymphocytes. Front Biosci. 2004;9:1000–1010. doi: 10.2741/1309. [DOI] [PubMed] [Google Scholar]
- Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4+ T cell responses in vivo and in vitro. Proceedings of the National Academy of Sciences. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage PA, Boniface JJ, Davis MM. A Kinetic Basis For T Cell Receptor Repertoire Selection during an Immune Response. Immunity. 1999;10:485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC Chemokine Receptor 5 Expression Defines Follicular Homing T Cells with B Cell Helper Function. J. Exp. Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KL, Plon SE. CHES1/FOXN3 interacts with Ski-interacting protein and acts as a transcriptional repressor. Gene. 2005;359:119–126. doi: 10.1016/j.gene.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T Cell Help in Generating Functional CD8 T Cell Memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proceedings of the National Academy of Sciences. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shunji Jia AM. Tob genes in development and homeostasis. Developmental Dynamics. 2007;236:913–921. doi: 10.1002/dvdy.21092. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Whitton JL. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat. Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- Stahl M, Dijkers PF, Kops GJPL, Lens SMA, Coffer PJ, Burgering BMT, Medema RH. The Forkhead Transcription Factor FoxO Regulates Transcription of p27Kip1 and Bim in Response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- Sun JC, Bevan MJ. Defective CD8 T Cell Memory Following Acute Infection Without CD4 T Cell Help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JC, Lehar SM, Bevan MJ. Augmented IL-7 Signaling during Viral Infection Drives Greater Expansion of Effector T Cells but Does Not Enhance Memory. J. Immunol. 2006;177:4458–4463. doi: 10.4049/jimmunol.177.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Stipdonk MJB, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- Vietor I, Huber LA. Role of TIS7 family of transcriptional regulators in differentiation and regeneration. Differentiation. 2007 doi: 10.1111/j.1432-0436.2007.00205.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and Type-I IFN Synergize for IFN- Production by CD4 T Cells, Whereas Neither Are Required for IFN- Production by CD8 T Cells after Listeria monocytogenes Infection. J Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire JK, Benning N, Whitton JL. Precursor Frequency, Nonlinear Proliferation, and Functional Maturation of Virus-Specific CD4+ T Cells. J Immunol. 2006;176:3028–3036. doi: 10.4049/jimmunol.176.5.3028. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Shortening the Infectious Period Does Not Alter Expansion of CD8 T Cells but Diminishes Their Capacity to Differentiate into Memory Cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and Memory CTL Differentiation. Annual Review of Immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. Eur J Immunol. 2006;36:1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Lara-Tejero M, Ploss A, Leiner I, Pamer EG. Rapid Development of T Cell Memory. J. Immunol. 2004;172:7239–7245. doi: 10.4049/jimmunol.172.12.7239. [DOI] [PubMed] [Google Scholar]
- Yamagishi N, Ishihara K, Saito Y, Hatayama T. Hsp105 family proteins suppress staurosporine-induced apoptosis by inhibiting the translocation of Bax to mitochondria in HeLa cells. Experimental Cell Research. 2006;312:3215–3223. doi: 10.1016/j.yexcr.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu L-G, Han K-J, Shu H-B. Identification of a ZU5 and Death Domain-containing Inhibitor of NF-{kappa}B. J. Biol. Chem. 2004;279:17819–17825. doi: 10.1074/jbc.M310737200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.