Abstract
Peroxynitrite-dependent formation of nitrotyrosine has been associated with inactivation of various enzymes and proteins possessing functionally important tyrosines. We have previously reported an enzymatic activity modifying the nitrotyrosine residues in nitrated proteins. Here we are describing a nonenzymatic reduction of nitrotyrosine to aminotyrosine, which depends on heme and thiols. Various heme-containing proteins can mediate the reaction, although the reaction also is catalyzed by heme. The reaction is most effective when vicinal thiols are used as reducing agents, although ascorbic acid also can replace thiols with lesser efficiency. The reaction could be inhibited by (z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1, but not other tested NO donors. HPLC with electrochemical detection analysis of the reaction identified aminotyrosine as the only reaction product. The reduction of nitrotyrosine was most effective at a pH close to physiological and was markedly decreased in acidic conditions. Various nitrophenol compounds also were modified in this reaction. Understanding the mechanism of this reaction could help define the enzymatic modification of nitrotyrosine-containing proteins. Furthermore, this also could assist in understanding the role of nitrotyrosine formation and reversal in the regulation of various proteins containing nitrotyrosine. It also could help define the role of nitric oxide and other reactive species in various disease states.
Keywords: aminotyrosine, nitric oxide
Nitric oxide (NO) is a signaling molecule generated from l-arginine via the catalytic action of both constitutive and inducible forms of NO synthases (1). A large body of evidence has been accumulated in the last decade establishing the role of NO in the pathogenesis of inflammatory, infectious, and degenerative human disease (2). The detrimental effects ascribed to NO often arise from its conversion to more reactive species through reactions with partially reduced oxygen (3).
Recent evidence supports a role for peroxynitrite in cellular damage and apoptosis (4–8) that was previously attributed entirely to NO. The formation of peroxynitrite from nitric oxide and superoxide anion is almost diffusion-limited (9). Peroxynitrite has been shown to cause lipid peroxidation (10–12), chemical cleavage of DNA (7, 13, 14), inactivation of key metabolic enzymes such as aconitase (15), ribonucleotide reductase, succinate dehydrogenase, and cytochrome oxidase of the mitochondrial electron transport chain (16–19). Peroxynitrite can also nitrate protein tyrosine residues, leading to decreased tyrosine phosphorylation by tyrosine kinases (20, 21) or to alteration of the functions of proteins/enzymes in vitro (22, 23) and perhaps in vivo (24, 25). It has been reported that nitrotyrosine formation in cytoskeletal components can alter their function (26). The present view is that protein nitration is associated with some toxic or deleterious effects of nitric oxide and with the interruption of cellular signaling processes. The formation of nitrotyrosine-containing proteins has been observed in neurodegenerative disease (27–29), acute lung injury (9, 30, 31), atherosclerosis (32–34), bacterial and viral infections (35–37), chronic inflammation (38–40), and endotoxin administration to animals (41).
We previously described an enzymatic activity in rat tissue that is capable of nitrotyrosine modification (42). We have tentatively called this enzymatic activity “nitrotyrosine denitrase” until the reaction and products are better defined. Such enzymatic activity could play an essential role in the protection or restoration of enzymatic and structural functions of the proteins containing nitrotyrosine. Here we present evidence for a nonenzymatic reduction of nitrotyrosine to aminotyrosine, which depends on heme and reducing agents. An understanding of this effect could provide useful information regarding the mechanisms leading to enzymatic “denitration” or provide methods to prevent nitrotyrosine formation and protein nitration in diverse pathophysiologic conditions.
Materials and Methods
Chemicals.
All chemicals were of research grade unless otherwise specified and were obtained from Sigma. Oxygen and argon gases were purchased from Aldrich, and methanol (HPLC grade) was purchased from EM Science. The water used was HPLC grade.
Nonenzymatic Modification.
Unless otherwise indicated, 100 nmol of nitrotyrosine (NT) or other nitrophenol compounds were incubated with 2.5 nmol of hemoglobin and 1 μmol of DTT in a final volume of 100 μl of PBS (pH 7.2), to obtain a solution of 1 mM NT, 25 μM hemoglobin, and 10 mM DTT. The mixture was boiled for 10 min unless otherwise indicated and was cooled, and 50 μl of 20% trichloroacetic acid (TCA) was added to the sample. Samples were subjected to 15,000 × g centrifugation for 30 min to precipitate proteins and macromolecules. The supernatant fractions were collected and diluted in the HPLC mobile phase buffer. Nitrotyrosine (480 pmol) was applied immediately to HPLC for analysis of nitrotyrosine content with an electrochemical detector.
HPLC-Electrochemical Analysis.
All samples were analyzed by HPLC equipped with two electrochemical detection channels. The optimal potentials for detection of tyrosine, aminotyrosine, and nitrotyrosine were determined by measuring the oxidative current over a range of electrode potentials. Based on the calibration, channel 1 was set to +0.6 V to quantify tyrosine and aminotyrosine and channel 2 was set to +0.85 V for the detection of nitrotyrosine. HPLC analysis was performed under isocratic conditions. For nitrotyrosine and tyrosine detection, an ODS 80-Tm C18 reverse phase analytical column (TosoHaas, Mongtomeryville, PA) was used with 50 mM sodium acetate, 50 mM sodium citrate, and 8% (vol/vol) methanol (pH 3.1) as the mobile phase. To quantify the aminotyrosine, 20 mM potassium phosphate and 10% (vol/vol) methanol (pH 7.5) was used with the same column. The amounts of tyrosine, nitrotyrosine, and aminotyrosine were estimated from the height of each peak and comparison to established tyrosine, nitrotyrosine, or aminotyrosine standards. Internal standards of each compound also were used to determine recoveries with the method.
Spectrophotometric Measurement of Nitroaromatic Compounds.
All samples were prepared as described above except that, after TCA precipitation, the supernatant fractions were mixed with 150 μl of 1M Tris⋅base (pH ≈10.9) and were adjusted to a final volume of 1 ml with water. Spectrophotometric measurement for the nitrophenol compound was conducted at the following predetermined wavelength for maximum absorbance: para-nitroaniline (379 nm); para-nitrocatechol and ortho-nitrotyrosine (427 nm); ortho-nitrophenol (416 nm); meta-nitrophenol (389 nm); para-nitrophenol and BOC-ortho-nitrotyrosine (405 nm). All experiments were repeated on at least three occasions, and representative data are presented.
Results
Nonenzymatic Modification of Nitrotyrosine.
We previously demonstrated the presence of an enzymatic activity in rat spleen homogenates capable of removing or modifying the nitro group of nitrotyrosine on nitrated BSA or other nitrotyrosine-containing proteins (42). We noticed that the amount of detected nitrotyrosine decreased considerably if the assay was stopped by Laemmli buffer and boiling (Y.K. and F.M., unpublished observation). This effect was independent of the incubation time and appeared to be very rapid. However, if the reaction was stopped by boiling with nonreducing Laemmli buffer, the decrease in nitrotyrosine content depended on incubation time of nitrotyrosine containing BSA with spleen homogenate, displaying the characteristics of the mentioned enzymatic reaction (42). In the present studies, we have investigated the nature of the nonenzymatic modification of nitrotyrosine.
We initially examined the changes in free nitrotyrosine content after incubation with spleen homogenates from lipopolysaccharide-treated animals in the presence of 10 mM DTT as a reducing agent. The samples were incubated for 10 min at 37°C or 100°C and then were precipitated with TCA. Nitrotyrosine from the supernatant fractions was quantified by HPLC/electrochemical detection (ECD) as described in Material and Methods. We found that the amounts of nitrotyrosine decreased considerably if the samples were boiled in the presence of DTT (Table 1). When homogenates were substituted with hemoglobin, the effect was even more pronounced. In the absence of tissue extract, hemoglobin, DTT, or high temperature, the amounts of nitrotyrosine did not change significantly. Only incubation with both DTT and hemoglobin showed some moderate decrease in nitrotyrosine amounts (Table 1).
Table 1.
Nonenzymatic modification of nitrotyrosine
| Sample composition | Amounts of detected nitrotyrosine, pmol
|
|
|---|---|---|
| 37°C incubation | 100°C incubation | |
| Nitrotyrosine, 1 mM | 132 | 164 |
| Nitrotyrosine, 1 mM and DTT, 10 mM | 128 | 148 |
| Nitrotyrosine, 1 mM and spleen homogenates* | 125 | 149 |
| Nitrotyrosine, 1 mM, spleen homogenates and DTT, 10 mM | 125 | 22 |
| Nitrotyrosine, 1 mM and hemoglobin, 25 μM | 136 | 147 |
| Nitrotyrosine, 1 mM, hemoglobin, 25 μM, and DTT, 10 mM | 85 | <1 |
Nitrotyrosine (100 nmol) was incubated with 10 mM DTT and indicated amounts of tested compounds. An equivalent of 180 pmol of nitrotyrosine was applied on the HPLC and analyzed by ECD.
*Protein (50 μg). Data from a representative experiment are presented.
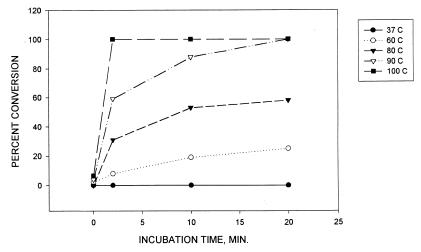
Next, the dynamics of nitrotyrosine conversion were studied. The effects of various temperatures and incubation times were tested as shown in Fig. 1. The limit of nitrotyrosine detection with this HPLC-ECD method was ≈1 pmol. The rate of nitrotyrosine conversion was considered 100% when the level of detected nitrotyrosine was <1 pmol. The reaction was more rapid with an increase in incubation temperature and was almost instantaneous at 100°C with almost 100% conversion whereas the percent of nitrotyrosine conversion at 37°C was close to zero.
Figure 1.
Dynamics of nitrotyrosine conversion. Nitrotyrosine (100 nmol) was incubated with 10 mM DTT and 25 μM hemoglobin at pH 7.2 at various temperatures and times as indicated. An equivalent of 180 pmol of nitrotyrosine was analyzed with HPLC-ECD as described in Materials and Methods. The percentage of conversion was calculated by the comparison with the amount of detected nitrotyrosine at time zero in a sample lacking hemoglobin. The detections of amounts <1 pmol were considered 100% conversion. A representative experiment is shown.
Nitrotyrosine Is Converted to Aminotyrosine.
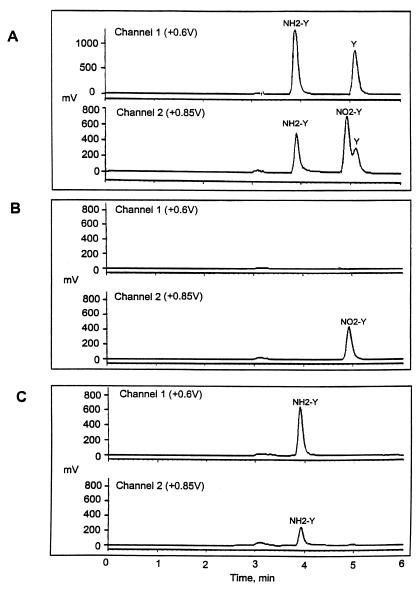
To identify the product of the nonenzymatic modification of nitrotyrosine, we performed an HPLC-ECD analysis of the reaction mixture with aminotyrosine and tyrosine as standards. After the incubation of free nitrotyrosine with hemoglobin in the presence of DTT, the samples were treated with TCA precipitation, and the amount of nitrotyrosine in supernatant fractions was estimated by HPLC-ECD as described in Material and Methods. A typical chromatogram is shown in Fig. 2. With the chromatographic conditions used, aminotyrosine was eluted from an ODS 80-Tm C18 reverse phase analytical column at 3.9 min, nitrotyrosine at 4.9 min, and tyrosine at 5.1 min (Fig. 2A). After incubation of nitrotyrosine with hemoglobin and DTT at 100°C, a new peak was detected with the retention time of 3.9 min. The peak was more pronounced in the channel 1 used for the detection of tyrosine and aminotyrosine (Fig. 2C). No other peaks were detected. The quantification of aminotyrosine obtained after the reaction revealed that an equivalent of 180 pmol of nitrotyrosine was reduced to equal amounts of aminotyrosine (Fig. 2C; some data not shown). This indicated that aminotyrosine was the only reaction product.
Figure 2.
Nitrotyrosine is reduced to aminotyrosine. (A) HPLC-ECD chromatogram showing the resolution of nitrotyrosine (NO2-Y), aminotyrosine (NH2-Y), and tyrosine (Y) standards. (B) A representative chromatogram of the reaction products after nitrotyrosine incubation with hemoglobin and DTT at room temperature or incubation at 100°C with either hemoglobin or DTT alone. (C) The chromatogram of the reaction products after nitrotyrosine incubation with hemoglobin and DTT at 100°C.
Nonenzymatic Nitrotyrosine Reduction is Heme-Dependent.
The rate of nitrotyrosine conversion depended on the concentration of hemoglobin (Table 2). With 10 mM DTT and 25 μM hemoglobin, almost 100% of nitrotyrosine was converted. Not only hemoglobin but other heme-proteins also resulted in an efficient reduction of nitrotyrosine (Table 2). We also tested some heme-related compounds. Both hemin and cytochrome C could mimic the hemoglobin effect with DTT; however, neither iron-deficient protoporphyrine IX nor ferric or ferrous iron alone could reduce the nitrotyrosine. Iron-reconstituted protoporphyrin IX mediated an efficient reaction whereas the mixture of pyrrole and iron was only a moderate catalyst. Interestingly, a nonheme compound vitamin B12 with DTT also catalyzed some conversion of nitrotyrosine, although with lower efficiency.
Table 2.
Heme-dependent reduction of nitrotyrosine
| Heme protein or compound | Nitrotyrosine conversion at 100°C, % |
|---|---|
| Hemoglobin, 25 μM | 100% |
| 2.5 μM | 100% |
| 0.25 μM | 73% |
| Myoglobin, 25 μM | 100% |
| Cytochrome C, 25 μM | 100% |
| Hemin, 25 μM | 100% |
| 2.5 μM | 100% |
| 0.25 μM | 24% |
| Protoporphyrin IX, 25 μM | 0% |
| Protoporphyrin IX with 25 μM Fe2+ | 100% |
| Fe3+, 25 μM | 0% |
| Fe2+, 25 μM | 0% |
| Pyrrole, 25 μM with Fe2+, 25 μM | 16% |
| Vitamin B12, 25 μM | 57% |
Nitrotyrosine (100 nmol) was incubated with 10 mM DTT and indicated amounts of tested compounds. An equivalent of 180 pmol of nitrotyrosine was applied on the HPLC and analyzed by ECD. The percentage of conversion was calculated by the comparison with amount of detected nitrotryosine in the sample lacking DTT and hemoglobin. Data from a representative experiment are presented.
Characterization of Nonenzymatic Reduction of Nitrotyrosine.
Reducing agents are also necessary for the conversion of nitrotyrosine to aminotyrosine. The effects of various DTT concentrations on the efficiency of nitrotyrosine conversion are shown in Table 3. At lower concentrations of DTT (0–3.6 mM), conversion was negligible, but, at 9 mM DTT, the reduction was complete. DTT was more potent than other reducing reagents tested. As shown in Table 3, neither cysteine nor glutathione facilitated the reaction. Low concentrations of β-mercaptoethanol were not effective, but at a concentration used in Laemmli buffer (350 mM), β-mercaptoethanol reduced 100% of nitrotyrosine. Interestingly, the nonthiol ascorbic acid also gave a moderate reduction. Thiol reacting and oxidizing agents such as diamide and mercuric chloride were effective inhibitors of the reaction.
Table 3.
Characterization of the nonenzymatic reduction of nitrotyrosine
| Compounds added | Nitrotyrosine conversion, % |
|---|---|
| Hemoglobin, 25 μM | |
| +9.0 mM DTT | 100% |
| +7.2 mM DTT | 40% |
| +3.6 mM DTT | 5% |
| +1.8 mM DTT | 0% |
| +0.9 mM DTT | 0% |
| Hemoglobin, 25 μM | |
| +100 mM cysteine | 0% |
| +100 mM glutathione | 0% |
| +10 mM β-mercaptoethanol | 0% |
| +350 mM β-mercaptoethanol | 100% |
| +100 mM ascorbic acid | 12% |
| Hemoglobin, 25 μM with 10 mM DTT | |
| +100 mM cystamine | 5% |
| +20 mM diamide | 0% |
| +20 mM mercuric chloride | 0% |
| Hemoglobin, 25 μM with 10 mM DTT | |
| +0.1 mM KCN | 100% |
| +0.1 mM SNP | 100% |
| +0.1 mM SNAP | 100% |
| +0.1 mM GSNO | 100% |
| +0.1 mM DETA-NO | 0% |
| Hemoglobin, 25 μM with 10 mM DTT | |
| pH 10 Glycine buffer | 70% |
| Borate buffer (10) | 87% |
| pH 7 Tricine buffer (7.0) | 100% |
| Phosphate buffer (7.2) | 100% |
| pH 4 Acetate buffer (4.0) | 10% |
| Citrate buffer (4.5) | 31% |
Nitrotyrosine (100 nmol) was incubated with indicated amounts of tested compounds. An equivalent of 180 pmol of nitrotyrosine was applied on the HPLC and analyzed by ECD. The percentage of conversion was calculated by the comparison with amount of detected nitrotyrosine in the sample lacking DTT and hemoglobin. Data from a representative experiment are presented. SNP, sodium nitroprusside; SNAP, S-nitroso-N-acetyl-d, l-penicillamine; DETA-NO, (z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino] diazen-1-ium-1, 2-diolate; GSNO, S-nitrosoglutathione.
Because the reaction depends on heme or heme-related compounds, we tested whether it could be inhibited by NO-donors or other reagents with affinity to heme. Surprisingly, such NO donors as sodium nitroprusside, S-nitroso-N-acetyl-d, l-penicillamine, or S-nitrosoglutathione were not effective at 100 μM. However, (z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl) amino]diazen-1-ium-1, 2-diolate at 0.1 mM completely inhibited the reaction with 25 μM hemoglobin and 10 mM DTT. In the presence of oxygen, the conversion of nitrotyrosine to aminotyrosine decreased by 15% (data not shown) whereas 0.1 mM sodium cyanide did not have any effect.
The reaction was pH-dependent. The conversion was maximal at close to physiological pH but was ineffective at low pH. At pH close to 10, the reaction efficiency decreased markedly (Table 3).
Nonenzymatic Conversion of Nitrophenol Compounds.
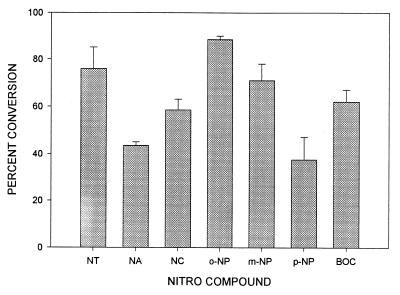
We next asked whether this reaction is specific to nitrotyrosine or could be observed with other nitrophenol compounds. For this purpose, we followed the conversion rate of different nitrophenol compounds spectrophotometrically. After reduction, the spectrum of each selected compound was changed, which allowed us to estimate the conversion efficiency. The measurements were conducted at predetermined wavelengths by using the maximum absorbance specific for each compound. We found that not only nitrotyrosine but also other nitrophenol compounds were converted in a 10-min reaction at 100°C (Fig. 3). The conversion rates for these compounds varied, but the compounds carrying the nitro-moiety in the ortho-position were converted with higher efficiency.
Figure 3.
Nonenzymatic conversion of various nitrophenol compounds. Each nitrophenol compound (1 mM for each) was incubated with 10 mM DTT and 25 μM hemoglobin at pH 7.2 at 100°C for 10 min. The conversion of each compound was analyzed spectrophotometrically at predetermined wavelengths as described in Materials and Methods. Data are presented as percent of conversion (means ± SE). NT, ortho-nitrotyrosine; NA, para-nitroaniline; NC, para-nitrocatechol; o-NP, ortho-nitrophenol; m-NP, meta-nitrophenol; p-NP, para-nitrophenol; BOC-NT, BOC-ortho-nitrotyrosine. The specific wavelengths are indicated in Material and Methods.
Discussion
Chemical nitration of tyrosine is a convenient tool for the identification of critical tyrosine residues in enzymes and proteins (43). Chemical nitration of functionally important tyrosine residues by tetranitromethane was often found to inactivate or alter the properties of the enzyme under investigation (44–46). It was only after the detection of in vivo nitrotyrosine formation in inflammatory conditions that the physiological aspects of nitrotyrosine metabolism came to attention.
Abundant production (1–120 μM) of nitrotyrosine was recorded under a number of pathological conditions such as rheumatoid arthritis (38), liver transplantation (47), septic shock (48), and amyotrophic lateral sclerosis (49). Reactive nitrogen species (2) and myeloperoxidase-dependent mechanisms (50, 51) are believed to be the main means of in vivo tyrosine nitration. The free amino acid form of nitrotyrosine has been shown to clear from the circulation with a half-life of 100 min (52). However, the mechanisms underlying these clearance processes are not known.
We previously reported that antinitrotyrosine antibodies detected a decrease in nitrotyrosine content of NT-containing BSA and other nitrated proteins after incubation with spleen homogenates (42). Careful examination of this phenomenon demonstrated the presence of an enzymatic activity that is capable of removing or modifying the nitro group on nitrotyrosine containing BSA (42). We have tentatively called this enzymatic activity “nitrotyrosine denitrase,” until the mechanism and the reaction products are determined. During our investigation, we noticed that the amount of nitrotyrosine detected by Western immunoblotting decreased considerably if the incubations were stopped by the addition of Laemmli buffer and boiling (Y.K. and F.M., unpublished observation). This effect was independent of the incubation time and appeared to be very rapid. However, if the reaction was stopped by boiling with nonreducing Laemmli buffer, the decrease in nitrotyrosine content depended on incubation time of NT-BSA and spleen homogenate, displaying the characteristics of the mentioned enzymatic reaction (42). In these present studies, we have investigated the nonenzymatic modification of nitrotyrosine in reducing conditions.
Nonenzymatic reduction of nitrotyrosine to aminotyrosine was found to strictly depend on the presence of heme and reducing reagents. The reaction rates are high at elevated temperatures and very low at room temperature (data not shown) or 37°C (Table 1 and Fig. 1). The amounts of detected free nitrotyrosine by HPLC-ECD were usually lower than the 180 pmol used in the reaction. This could be explained by decreased solubility of nitrotyrosine in acidic conditions after TCA treatment, which results in losses during centrifugation. Higher yields after incubation of nitrotyrosine at 100°C, which could increase the solubility of nitrotyrosine before TCA treatment, support this hypothesis. A moderate reduction of nitrotyrosine was observed when incubated with 25 μM hemoglobin and 10 mM DTT at 37°C (Table 1, 85 pmol detected versus 132 pmol recovered when nitrotyrosine was incubated alone). The lower concentration of heme proteins in spleen homogenate explains why no significant decrease in nitrotyrosine content is detected when incubated in the presence of DTT at 37°C (Table 1, 125 versus 132 pmol). Incubation at 100°C with spleen homogenate showed a significant reduction of nitrotyrosine (Table 1, 22 versus 164 pmol) whereas incubation with hemoglobin and DTT under these conditions reduced all of the nitrotyrosine (Table 1). Analysis of the reaction products indicated that only aminotyrosine could be detected (Fig. 2). However, the reduction of the nitrogroup is a two-step process with the formation of a nitroso intermediate. The conversion rate from nitroso- to aminotyrosine under the conditions of nonenzymatic reduction is possibly too rapid for the detection of the intermediate by the employed method. Other nitrophenol compounds were also substrates in this reaction, as indicated in Fig. 3.
The reaction was catalyzed not only by hemoglobin but also by other heme-containing proteins (Table 2). Free heme also catalyzed the reaction, although the effective concentrations were higher (Table 2). Protoporphyrin IX, ferric, or ferrous irons alone were not effective (Table 2), but iron reconstituted protoporphyrin IX was. Thus, nitrotyrosine reduction in this reaction depends on the presence of coordinated iron. This conclusion is supported by a moderate reduction of nitrotyrosine by a mixture of pyrrole and ferrous iron [≈16% reduction (Table 2)]. In this case, the pyrrole rings are not covalently bound but can form a complex with ferrous iron similar to the iron coordination in heme. The heme-coordinated iron can be substituted by corrin-coordinated cobalt in vitamin B12, which exhibited a significant catalytic activity [57% nitrotyrosine reduction (see Table 2)]. It is possible that other coordinated transition metals can be effective catalysts for nonenzymatic reduction of nitrotyrosine and other nitrophenol compounds.
Nitrotyrosine reduction strictly depended on reducing agents, but not all reducing agents displayed the same efficiency. Of several thiol reducing agents, such as DTT, glutathione, cysteine, and β-mercaptoethanol, only DTT was effective at a concentration of 10 mM. β-mercaptoethanol was not effective at 10 mM but, at the 350 mM concentration used in Laemmli buffer, was able to convert 100% of nitrotyrosine to aminotyrosine. It appears that vicinal thiols are more effective in the reduction of nitrotyrosine. These data explain why under the reducing conditions of Laemmli buffer nitrotyrosine from nitrotyrosine containing BSA was converted much faster than expected from an enzymatic reaction (Y.K. and F.M., unpublished observation). Heme proteins from tissue homogenates in the presence of β-mercaptoethanol are most probably reducing nitrotyrosine to aminotyrosine. Such a reaction prevents nitrotyrosine-specific antibody to recognize the reduced epitope. Nitrotyrosine serves as a biochemical marker of peroxynitrite-induced damage. Immunohistochemical approaches have been widely used for detection of the in vivo formation and accumulation of nitrotyrosine (53). The modification of nitrotyrosine under the reducing conditions of Laemmli buffer reported here should lead to an underestimation of nitrotyrosine amounts in the sample. This could explain the difference between the few studies reporting the detection of nitrotyrosine by Western immunoblotting of tissue homogenates (54) and the vast literature on immunohistochemical detection of nitrotyrosine in tissue sections (53).
We were able to inhibit the nonenzymatic reduction of nitrotyrosine by addition of either oxidized forms of reducing agents, such as cystamine, or thiol oxidizing agents such as diamide or mercuric chloride (Table 3). The addition of potassium cyanide also did not affect the reduction of nitrotyrosine. However, taking into consideration that vitamin B12, which contains a coordinated cobalt ion in cyanide form, replaced heme with a moderated efficiency (Table 2), it is possible than some forms of cyanide complexed iron are effective in the reaction of nitrotyrosine reduction. We speculate that heme iron in this reaction is engaged in a complex formation with nitrotyrosine and does not participate in electron donation. Only the NO donor (z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1, 2-diolate was able to inhibit the aminotyrosine formation whereas S-nitrosoglutathione, sodium nitroprusside, and S-nitroso-N-acetyl-d, l-penicillamine were not effective. Differences in the stability of the compounds, in the dynamics of NO generation, or in NO species generated by these donors could account for the discrepancy in the inhibitory properties of these donors. Additional studies should be able to determine the mechanism of (z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1, 2-diolate-dependent inhibition of nitrotyrosine reduction. The formation of aminotyrosine is most effective at a physiologic pH whereas at acidic pH, the reaction is not effective (Table 3). Thus, the nonenzymatic reduction of nitrotyrosine in samples containing heme proteins can be prevented by acidification of the sample and addition of (z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1, 2-diolate during their processing for SDS/PAGE. The same effect can be obtained by oxidation/omission of the reducing agents or their replacement with glutathione or cysteine.
The nonenzymatic reduction of nitrotyrosine described here may be related to the enzymatic modification of nitrotyrosine recently described by our laboratory (42). The understanding of the precise mechanism of the nonenzymatic reaction could shed light on the properties of the enzyme(s) that are able to modify nitrotyrosine in vivo.
Acknowledgments
We thank the John Dunn foundation and the University of Texas for their support to F.M. and the North Atlantic Treaty Organization for the fellowship to B.B.
Abbreviations
- NT
nitrotyrosine
- ECD
electrochemical detection
- TCA
trichloroacetic acid
References
- 1.Lane P, Gross S S. Semin Nephrol. 1999;19:215–229. [PubMed] [Google Scholar]
- 2.Patel R P, McAndrew J, Sellak H, White C R, Jo H, Freeman B A, Darley-Usmar V M. Biochim Biophys Acta. 1999;1411:385–400. doi: 10.1016/s0005-2728(99)00028-6. [DOI] [PubMed] [Google Scholar]
- 3.Eiserich J P, Patel R P, O’Donnell V B. Mol Aspects Med. 1998;19:221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 4.Estevez A G, Radi R, Barbeito L, Shin J T, Thompson J A, Beckman J S. J Neurochem. 1995;65:1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin K T, Xue J Y, Nomen M, Spur B, Wong P Y. J Biol Chem. 1995;270:16487–16490. doi: 10.1074/jbc.270.28.16487. [DOI] [PubMed] [Google Scholar]
- 6.Troy C M, Derossi D, Prochiantz A, Greene L A, Shelanski M L. J Neurosci. 1996;16:253–261. doi: 10.1523/JNEUROSCI.16-01-00253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo C, Ohshima H. Nitric Oxide. 1997;1:373–385. doi: 10.1006/niox.1997.0143. [DOI] [PubMed] [Google Scholar]
- 8.Haendeler J, Zeiher A M, Dimmeler S. Vitam Horm (San Francisco) 1999;57:49–77. doi: 10.1016/s0083-6729(08)60640-8. [DOI] [PubMed] [Google Scholar]
- 9.Haddad I Y, Pataki G, Hu P, Galliani C, Beckman J S, Matalon S. J Clin Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radi R, Beckman J S, Bush K M, Freeman B A. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 11.Rubbo H. Medicina. 1998;58:361–366. [PubMed] [Google Scholar]
- 12.Hogg N, Kalyanaraman B. Biochim Biophys Acta. 1999;1411:378–384. doi: 10.1016/s0005-2728(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 13.Inoue S, Kawanishi S. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- 14.Markesbery W R. Free Radical Biol Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 15.Castro L, Rodriguez M, Radi R. J Biol Chem. 1994;269:29409–29415. [PubMed] [Google Scholar]
- 16.Radi R, Rodriguez M, Castro L, Telleri R. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 17.Cassina A, Radi R. Arch Biochem Biophys. 1996;328:309–316. doi: 10.1006/abbi.1996.0178. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe M A, Cooper C E. J Biol Chem. 1998;273:30961–30972. doi: 10.1074/jbc.273.47.30961. [DOI] [PubMed] [Google Scholar]
- 19.Brown G C. Biochim Biophys Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 20.Gow A J, Duran D, Malcolm S, Ischiropoulos H. FEBS Lett. 1996;385:63–66. doi: 10.1016/0014-5793(96)00347-x. [DOI] [PubMed] [Google Scholar]
- 21.Brito C, Naviliat M, Tiscornia A C, Vuillier F, Gualco G, Dighiero G, Radi R, Cayota A M. J Immunol. 1999;162:3356–3366. [PubMed] [Google Scholar]
- 22.Roberts E S, Lin H, Crowley J R, Vuletich J L, Osawa Y, Hollenberg P F. Chem Res Toxicol. 1998;11:1067–1074. doi: 10.1021/tx980099b. [DOI] [PubMed] [Google Scholar]
- 23.Zou M, Yesilkaya A, Ullrich V. Drug Metab Rev. 1999;31:343–349. doi: 10.1081/dmr-100101922. [DOI] [PubMed] [Google Scholar]
- 24.MacMillan-Crow L A, Crow J P, Kerby J D, Beckman J S, Thompson J A. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacMillan-Crow L A, Crow J P, Thompson J A. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 26.Eiserich J P, Estevez A G, Bamberg T V, Ye Y Z, Chumley P H, Beckman J S, Freeman B A. Proc Natl Acad Sci USA. 1999;96:6365–6370. doi: 10.1073/pnas.96.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Good P F, Werner P, Hsu A, Olanow C W, Perl D P. Am J Pathol. 1996;149:21–28. [PMC free article] [PubMed] [Google Scholar]
- 28.Good P F, Hsu A, Werner P, Perl D P, Olanow C W. J Neuropathol Exp Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Boven L A, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet H S. J Immunol. 1999;162:4319–4327. [PubMed] [Google Scholar]
- 30.Kooy N W, Royall J A, Ye Y Z, Kelly D R, Beckman J S. Am J Respir Crit Care Med. 1995;151:1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- 31.Kristof A S, Goldberg P, Laubach V, Hussain S N. Am J Respir Crit Care Med. 1998;158:1883–1889. doi: 10.1164/ajrccm.158.6.9802100. [DOI] [PubMed] [Google Scholar]
- 32.Beckmann J S, Ye Y Z, Anderson P G, Chen J, Accavitti M A, Tarpey M M, White C R. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 33.Buttery L D, Springall D R, Chester A H, Evans T J, Standfield E N, Parums D V, Yacoub M H, Polak J M. Lab Invest. 1996;75:77–85. [PubMed] [Google Scholar]
- 34.Hogg N, Darley-Usmar V M, Graham A, Moncada S. Biochem Soc Trans. 1993;21:358–362. doi: 10.1042/bst0210358. [DOI] [PubMed] [Google Scholar]
- 35.Adler H, Beland J L, Del-Pan N C, Kobzik L, Brewer J P, Martin T R, Rimm I J. J Exp Med. 1997;185:1533–1540. doi: 10.1084/jem.185.9.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giorgio S, Linares E, Capurro M d L, de Bianchi A G, Augusto O. Photochem Photobiol. 1996;63:750–754. doi: 10.1111/j.1751-1097.1996.tb09626.x. [DOI] [PubMed] [Google Scholar]
- 37.Facchetti F, Vermi W, Fiorentini S, Chilosi M, Caruso A, Duse M, Notarangelo L D, Badolato R. Am J Pathol. 1999;154:145–152. doi: 10.1016/S0002-9440(10)65261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaur H, Halliwell B. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 39.Kimura H, Hokari R, Miura S, Shigematsu T, Hirokawa M, Akiba Y, Kurose I, Higuchi H, Fujimori H, Tsuzuki Y, et al. Gut. 1998;42:180–187. doi: 10.1136/gut.42.2.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn B, Han B S, Kim D J, Ohshima H. Carcinogenesis. 1999;20:1337–1344. doi: 10.1093/carcin/20.7.1337. [DOI] [PubMed] [Google Scholar]
- 41.Bian K, Davis K, Kuret J, Binder L, Murad F. Am J Physiol. 1999;277:F33–F40. doi: 10.1152/ajprenal.1999.277.1.F33. [DOI] [PubMed] [Google Scholar]
- 42.Kamisaki Y, Wada K, Bian K, Balabanli B, Davis K, Martin E, Behbod F, Lee Y C, Murad F. Proc Natl Acad Sci USA. 1998;95:11584–11589. doi: 10.1073/pnas.95.20.11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prieels J P, Dolmans M, Leonis J, Brew K. Eur J Biochem. 1975;60:533–539. doi: 10.1111/j.1432-1033.1975.tb21032.x. [DOI] [PubMed] [Google Scholar]
- 44.Feste A, Gan J C. J Biol Chem. 1981;256:6374–6380. [PubMed] [Google Scholar]
- 45.De Caro J D, Behnke W D, Bonicel J J, Desnuelle P A, Rovery M. Biochim Biophys Acta. 1983;747:253–262. doi: 10.1016/0167-4838(83)90104-8. [DOI] [PubMed] [Google Scholar]
- 46.Froschle M, Ulmer W, Jany K D. Eur J Biochem. 1984;142:533–540. doi: 10.1111/j.1432-1033.1984.tb08318.x. [DOI] [PubMed] [Google Scholar]
- 47.Skinner K A, Crow J P, Skinner H B, Chandler R T, Thompson J A, Parks D A. Arch Biochem Biophys. 1997;342:282–288. doi: 10.1006/abbi.1997.0114. [DOI] [PubMed] [Google Scholar]
- 48.Wizemann T M, Gardner C R, Laskin J D, Quinones S, Durham S K, Goller N L, Ohnishi S T, Laskin D L. J Leukocyte Biol. 1994;56:759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]
- 49.Bruijn L I, Beal M F, Becher M W, Schulz J B, Wong P C, Price D L, Cleveland D W. Proc Natl Acad Sci USA. 1997;94:7606–7611. doi: 10.1073/pnas.94.14.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eiserich J P, Hristova M, Cross C E, Jones A D, Freeman B A, Halliwell B, van der Vliet A. Nature (London) 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 51.Sampson J B, Ye Y, Rosen H, Beckman J S. Arch Biochem Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- 52.Kamisaki Y, Wada K, Ataka M, Yamada Y, Nakamoto K, Ashida K, Kishimoto Y. Biochim Biophys Acta. 1997;1362:24–28. doi: 10.1016/s0925-4439(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 53.Viera L, Ye Y Z, Estevez A G, Beckman J S. Methods Enzymol. 1999;301:373–381. doi: 10.1016/s0076-6879(99)01101-5. [DOI] [PubMed] [Google Scholar]
- 54.MacMillan-Crow L A, Thompson J A. Methods Enzymol. 1999;301:135–145. doi: 10.1016/s0076-6879(99)01076-9. [DOI] [PubMed] [Google Scholar]