Abstract
The 2005 completion of the entire genome sequence of the 1918 H1N1 pandemic influenza virus represents both a beginning and an end. Investigators have already begun to study the virus in vitro and in vivo to better understand its properties, pathogenicity, transmissibility and elicitation of host responses. Although this is an exciting new beginning, characterization of the 1918 virus also represents the culmination of over a century of scientific research aiming to understand the causes of pandemic influenza. In this brief review we attempt to place in historical context the identification and sequencing of the 1918 virus, including the alleged discovery of a bacterial cause of influenza during the 1889–1893 pandemic, the controversial detection of ‘filter-passing agents’ during the 1918–1919 pandemic, and subsequent breakthroughs in the 1930s that led to isolation of human and swine influenza viruses, greatly influencing the development of modern virology.
Introduction
The announcement in 2005 that a virus causing fatal influenza during the great influenza pandemic of 1918–1919 had been sequenced in its entirety [1], in the laboratory of co-author JKT, has prompted renewed interest in the 1918 virus. The ongoing H5N1 avian influenza epizootic, and the possibility that it might also cause a pandemic [2], increase the importance of understanding what happened in 1918. However, in reviewing the scientific approach to unlocking an old puzzle, it is important to note that the sequencing of the 1918 virus took place after more than century of exhaustive and sometimes disheartening efforts to discover the cause of influenza (Figure 1). Indeed, the influenza search not only pre-dated the great pandemic of 1918, but also attracted the efforts of some of the greatest researchers of the 19th and 20th centuries. Along the way, the new fields of bacteriology and virology were advanced, and a productive marriage between microbiology, epidemiology and experimental science began. In describing here the 10-year effort (1995–2005) to sequence the genome of the 1918 pandemic influenza virus, we attempt also to place it within this important historical perspective.
Figure 1.
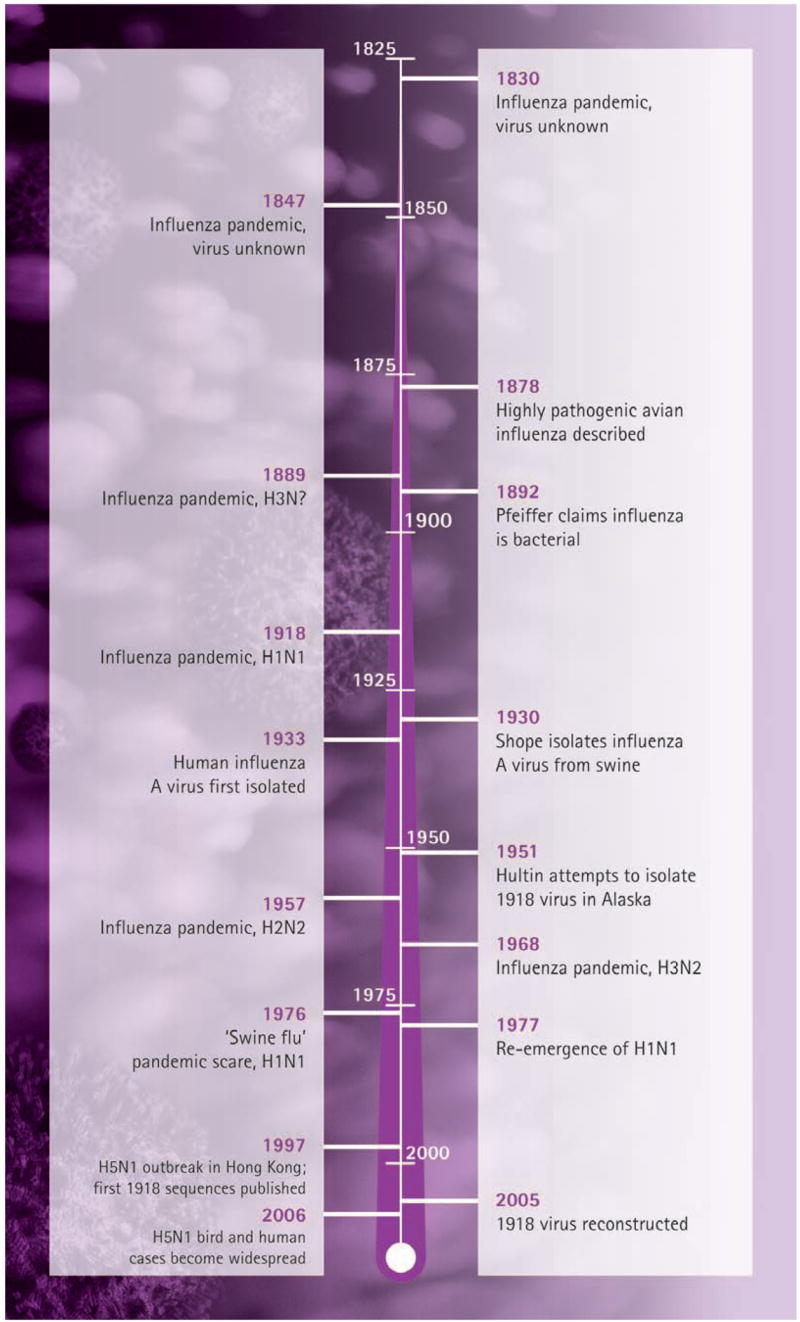
A timeline of key dates in the history of influenza occurrence and characterization
Framing influenza as a clinical and epidemiological entity
In the pre-microbiological era (before 1876) there was much confusion about the causes of both the common communicable diseases like colds, smallpox, and measles, and the great epidemic diseases like cholera and plague. Epidemiological notions of carriers and intermediate vectors were not easily envisioned, preventing many observers from accepting a unified concept of infectious diseases, some of which were directly transmissible from person to person (for example, smallpox), others of which were acquired from intermediate vector hosts (for example, plague), still others of which were acquired from contaminated environmental sources (for example, cholera) or were acquired by more than one of the mechanisms just noted (for example, smallpox and cholera).
In the pre-microbial era, influenza had been considered among the more confusing of the pandemic diseases for several reasons: (i) the signs and symptoms were non-specific, leading to confusion with other conditions; (ii) interpandemic influenza-like illnesses were often attributed to other upper respiratory infections (URIs), fuelling ongoing debates about whether interpandemic influenza existed at all, or whether URIs were the same thing as interpandemic influenza; (iii) pandemics occurred sporadically and unpredictably, prompting debates about whether a new influenza-like disease was the same as the one seen decades ago; and (iv) mortality statistics, which began to appear in developed nations in the mid-1800s, showed high mortality only for the first year or two of a pandemic, seeming to support the belief that interpandemic influenza did not occur.
Each of four major influenza pandemics before 1918, however, incrementally advanced scientific understanding of the disease. The 1781–1782 pandemic evolved so rapidly, and was so widespread, that explosive outbreaks of febrile respiratory illness in the general population alone became a distinguishing characteristic, supporting the notion of a specific disease entity. The 1789–1799 pandemic, coming soon thereafter, emphasized this recently learned lesson. The 1830–1833 pandemic, though less impressive, arrived at about the same time as the second cholera pandemic (1831–1833), of which the inevitable march toward Europe was followed for over a year in the daily newspapers, helping to establish a deep popular epidemiological concept of pandemic diseases. The influenza pandemic of 1847–1851 nearly coincided with the next (third) cholera pandemic (1849–1855): this was the pandemic in which British epidemiologist John Snow (1813–1858) first characterized the epidemiology and waterborne transmission of cholera [3]. The 1847–1851 influenza pandemic was also the first to occur in an era of national vital statistics: the British disease registry, set up in 1836, was able to characterize the general epidemiological pattern of influenza mortality for the first time.
After the 1847–1851 pandemic, however, there was little recognizable influenza in succeeding decades, and little evidence in the mortality records that people in the expected risk age groups, infants and the elderly, were dying from any respiratory disease. If not forgotten, pandemic influenza became by the late 1880s a curious memory related by elder physicians to young medical students and house staff. Then, in 1889, the most explosive and widespread influenza pandemic up to that time appeared suddenly and returned with uncharacteristic perseverance for several years thereafter, causing as many as five successive annual mortality peaks between 1889 and 1894 [4]. However, the 1889 pandemic (now thought to have been caused by an influenza A virus with an H3 subtype haemagglutinin [HA], as determined by archaeserology [5] and supported by recent archaevirology; see below) also occurred in an era ready to study it scientifically. By that time virtually all developed nations had vital statistics systems in place, and thus the levels and pattern of mortality could be documented. More importantly, biomedical research had just entered the microbiological era [6]. By 1889, in the middle of an age of great scientific excitement and progress, researchers were ready to apply their experiences to discovering the cause of an important disease, influenza, for which there had been no clinical material available for nearly 35 years.
A reputed cause of influenza identified in 1892
It was in 1892 that the venerated physician/bacteriologist Richard Friedrich Johannes Pfeiffer (1858–1945), in partnership with physician/bacteriologist Shibasaburo Kitasato (1852–1931), both working in Berlin under Robert Koch (1843–1910), reported the discovery of a new bacterium [7], which Kitasato was able to cultivate and sustain on artificial media [8], and which both scientists claimed to be the cause of pandemic influenza. When their initial, brief, 1892 report was followed up by more extensive data in 1893 [9], the scientific world was taken by storm; it seemed to many that Pfeiffer had taken all of the necessary steps to establish Bacillus influenzae, as he called it, as the true aetiological agent of influenza. (B. influenzae was usually referred to as ‘Pfeiffer’s bacillus’ in the literature of the late 19th and early 20th centuries, and is now known as Haemophilus influenzae).
However, although B. influenzae was clearly a pathogenic organism, and was often cultured from fatal cases of influenza, other investigators were unable to confirm Pfeiffer’s strong association, a problem compounded by the apparent disappearance of pandemic influenza within a year or two of Pfeiffer’s discovery. The verdict was unclear, but the notion that B. influenzae was the true cause of influenza persisted up to the time of the next pandemic in 1918 (see below), when Rockefeller scientists Peter Kosciusko Olitsky (1886–1964) and Frederick L Gates (1886–1933) provided strong evidence against a causal association, documenting that the infective influenza agent survived passage through filters that excluded B. influenzae [10].
Despite this bacterial blind alley, it is important to note that most of the deaths during the 1918–1919 influenza pandemic were associated with secondary bacterial invaders (for review of clinical and pathological features of the 1918 pandemic see [11]), among them H. influenzae, which Pfeiffer had discovered. Pfeiffer, a budding 38-year-old researcher at the time of his discovery, went on to have a long and distinguished career as an originator of typhoid vaccination, the discoverer of bacteriolysis (‘Pfeiffer’s phenomenon’), a conceptualizer of endotoxin, the discoverer of the pathogenic organism Micrococcus (now Moraxella) catarrhalis, and a tropical disease investigator of plague (in India) and malaria (in Italy; [12–14]). Kitasato, who had already discovered the cause of tetanus (1889) and had co-developed, with Emil von Behring (1854–1917), both tetanus and diphtheria antitoxins in 1890 [15,16], went on to co-discover the bacterial cause of plague in 1894 [17], and to support his protégé Kiyoshi Shiga (1871–1957) in elucidating the cause of shigellosis in 1898.
Interpandemic advances in virology (1892–1918)
The field of virology can be said to have been born in 1892, the same year in which Pfeiffer published his claim for B. influenzae as the cause of influenza [7]. Before that time the word virus had for many decades been used non-specifically to describe a hypothetical communicable agent, without denoting any particular size, morphology or physical characteristics. By the 1890s most communicable diseases were assumed to be caused by bacteria, and establishing causality required culturing them on artificial media. In the 1880s Louis Pasteur (1822–1895) had failed to isolate the causative agent of rabies, but when an effective vaccine was produced few doubted that rabies was caused by a bacterium that, for whatever reason, had not yet been cultivated. Then, in 1892, the young Russian botanist Dmitrii Ivanovski (1864–1940) showed that tobacco mosaic disease was caused by an unseen agent that passed through filters with pores too small to admit bacteria [18]. Six years later, in 1898, the Dutch botanist/microbiologist Martinus Willem Beijerinck (1851–1931) showed that this agent could be serially passed in a manner that indicated it was a replicating agent, with replication occurring only in living plant tissue [19]. Presciently, Beijerinck speculated on the existence and mechanism of replication of what we now call viruses, writing that ‘the contagium, in order to reproduce, must be incorporated into the living protoplasm of the cell, into whose reproduction it is, in a manner of speaking, passively drawn’ [20].
By the turn of the 19th/20th century, Chamberland and Berkfeld filters were being manufactured and used in research laboratories, allowing microbiologists to filter infectious fluids to remove bacteria that were presumably too large to pass through their pores. Using this technology, a variety of ‘filter-passing’ agents were identified in short order, including the agents of foot and mouth disease of cattle (1897–1898; [21,22]), bovine pleuro-pneumonia [23], rabbit myxomatosis [24], and African horse sickness (1900, [25]). An ever-increasing number of filter-passing agents were soon linked to many other plant, animal and human diseases: in 1903 Émile Roux (1853–1953) counted nine of them [26] and by 1906 Paul Remlinger (1871–1964) had raised the number to 18 [27].
However, the situation was complicated by the discovery that not all filter-passing agents were uncultivatable in bacterial media. The agent of bovine pleuro-pneumonia, for example, was cultivated early on (it is now known to be a mycoplasma). In 1917, George B Foster Jr claimed that a filter-passing agent caused the common cold, even though he simultaneously cultivated ‘minute coccoid bodies’ and had to admit that he could not distinguish between these bodies and an ‘ultramicroscopic’ (undetected) virus as the true cause [28]. On the eve of the 1918 influenza pandemic, distinct concepts of viruses and bacteria as separate and fundamentally different infectious entities were not yet mature. According to historian Lise Wilkinson, this problem ‘delayed… the virus concept in the first decades of the [20th] century’ [20], and it undoubtedly complicated the picture when the 1918 pandemic appeared.
Advances in understanding avian influenza
At the time of the 1918 influenza pandemic, no-one suspected that the cause of the human disease was derived from an avian infectious agent. Strong associations between some human influenza epidemics and equine epizootics in the 19th century [29] had been noted, but a human–swine influenza link had not been established, and indeed was not to be noted until the detection of swine epizootics in China and the United States during the autumn 1918 wave of the influenza pandemic [30,31]. Highly pathogenic avian influenza (then called ‘fowl plague’) had been recognized as a disease entity since 1878 [32], but was not well known to physicians or biomedical researchers. Between 1901 and 1903, Italian and Austrian researchers, working independently, identified filterable agents as the cause of avian influenza [33–35]. (Of unexpected importance, one team even noted that epizootics in domestic chickens were associated with epizootics of pneumoenteritis in pigs, transmission of disease to pet birds, and onward from pet birds to humans [35]. It is also interesting to note, in light of contemporary concerns about the spread of H5N1 avian influenza [2] that a 1901 Austrian epizootic in domestic chickens had been linked to importation of pet birds from Italy [33].) Schäfer identified fowl plague virus as influenza A in 1955 [36]. Additional avian influenza A viruses were identified in the 1960s [37]. Webster and colleagues proposed that pandemic influenza viruses might be related to avian influenza viruses in 1967 [38]. Slemons isolated influenza A viruses from wild ducks in 1974 [39], and it is now generally agreed that wild aquatic birds are the natural reservoir for influenza A viruses (reviewed in [40]).
Research efforts to identify the cause of the 1918–1919 pandemic
As noted, at the time of the 1918 influenza pandemic, biomedical thinking about influenza was dominated by Richard Pfeiffer’s 1892 claim that B. influenzae was its cause [7]. Indeed, in 1918 Pfeiffer was still active and vocal in making the case for the organism he had discovered [41,42]. That it was not universally cultivated from all influenza cases did not discredit Pfeiffer’s claim, because B. influenzae was difficult to grow under the conditions of the day.
The majority of individuals who died during the 1918 pandemic succumbed to secondary bacterial pneumonia [43–45], caused by Streptococcus pneumoniae, Streptococcus pyogenes, H. influenzae, Staphylococcus aureus, and other organisms. Moreover, a subset died rapidly after the onset of symptoms, often with either massive acute pulmonary haemorrhage or pulmonary oedema, and often in fewer than 5 days. In the hundreds of autopsies performed in 1918, the primary pathological findings tended to be confined to the respiratory tree: death was due to pneumonia and respiratory failure. These findings are consistent with infection by a well adapted influenza virus capable of rapid replication throughout the entire respiratory tree with little clinical or pathological evidence for systemic virus infection [45].
The autumn wave of the 1918 pandemic proceeded so quickly that standardized research to investigate the cause could not easily be set up. Nevertheless, during the autumn and winter many researchers attempted to confirm or disprove Pfeiffer’s claims, the latter by looking for filter-passing agents, something not possible during the previous 1889 pandemic. The first to succeed, on 1 September 1918, were Nicolle and Lebailly, who claimed to have transferred disease to two healthy volunteers via filtered sputum from a patient in the third day of his illness [46]. Despite equivocal results from other investigators, between December 1918 and March 1919 Yamanouchi and colleagues seemed to confirm and extend the results of the French investigators in inoculation experiments with 24 human volunteers [47]. Six of these volunteers had recovered from influenza; the remaining 18 volunteers, who had not had detectable illness beforehand, all developed influenza-like symptoms after a 2–3 day incubation period, including those receiving filtered and unfiltered nasopharyngeal inoculations of pooled infectious sputum.
Negative or more equivocal results, as well as supporting results, were soon published [48–69] and an air of cautious scepticism prevailed. Although one reviewer could claim about human influenza in 1920 that ‘[i]t is perhaps fair to state that the trend of opinion has gradually been in favour of the theory that the primary infecting agent is a filter passer’ [61], many others, particularly clinicians sceptical of claims for invisible and hypothetical agents, did not agree, and the question of influenza aetiology remained open for another decade. By the early 1920s, annual influenza recurrences had died down, and influenza again became an indolent endemic winter disease. With little clinical material available, and perhaps with a desire to forget the horrors of the recent pandemic, influenza research quieted down and further attempts to elucidate the aetiology were left to but a few investigators.
Swine influenza and the discovery of porcine and human influenza viruses
It has often been true in science that breakthroughs come from unexpected quarters. In 1931 Rockefeller Institute investigator Richard E Shope (1901–1966) published the first three of a series of landmark papers [70–72] establishing the aetiology of ‘swine influenza’ or ‘hog flu’, the new epizootic disease of pigs that had been noted initially during the autumn wave of the 1918 influenza pandemic [30,31]. It is now believed that the pandemic virus appearing in 1918 was transmitted from humans to pigs, at that time splitting off into two lineages, one human, the other porcine [73] (reviewed in [74]). Both lineages persist today, the classical swine influenza lineage having evolved continually since 1918, and the human lineage having caused pandemic and endemic influenza from 1918 to 1956. The human line apparently disappeared entirely around 1957 only to reappear in 1977, after possible release from a freezer [75], and has continued to circulate endemically in humans up to the present time.
Shope’s studies were important in their own right, but perhaps more so because they stimulated American and British research groups to take up, once again, the search for the cause of human influenza. In 1933 Alphonse Raymond Dochez (1882–1964) and colleagues produced apparent influenza via human nasopharyngeal inoculation and succeeded in cultivating and serially passing a virus in primary chick embryo cultures, demonstrating that passage material still produced human disease [76]. Several weeks later a British group that had been collaborating with Dochez, led by Sir Christopher Howard Andrewes (1896–1988), who had trained at the Rockefeller Institute in the 1940s, Wilson Smith (1897–1965) and Sir Patrick Playfair Laidlaw (1881–1940), reported isolation and serial propagation of human influenza virus in ferrets [77], introducing the great advantage of both a living culture medium and an animal model. (The human virus was found to cause a catarrhal disease in ferrets after a 2-day incubation period.) The papers of these two influential groups, along with the ongoing work of Shope and colleagues [70–72], led to an explosion of research in the field of virology, which has continued unabated until the present time.
The modern characterization of the influenza A virus
The work of Shope, of Dochez and colleagues, and of the Mill Hill group led by Andrewes, Smith and Laidlaw, resulted in the publication of hundreds of research papers during the 1930s, making influenza the most studied and best understood viral disease of its time. This body of work is too voluminous to review here, but it can be said that efforts to characterize influenza were a driving force behind the development of whole fields of investigation and new research methods, including virology, serology and immunology, experimental animal models, and modern vaccinology and passive immunotherapy [78,79]. By the 1940s influenza B and distinctive strains of influenza A had been identified, vaccines and immune serums had been produced and tested, and a generation of young scientists had been stimulated to embark on careers in virology, among them Thomas Francis (1900–1969), who did perhaps more than any other scientist to characterize influenza, Sir Charles Stuart-Harris (1909–1997), who joined the Mill Hill group and worked productively with many British and American colleagues, Sir Frank Macfarlane Burnet (1899–1985), the great Russian virologist Anatolii Smorodintsev (1901–1986), Maurice Hilleman (1919–2005), and even Jonas Salk (1914–1995), whose early work with influenza vaccines [80] proved to be important in his development, more than a decade later, of the first widely used poliomyelitis vaccine. By 1950, virology had truly come of age, and two generations of scientists could look back on the tragedy of the 1918 influenza pandemic with the seemingly impossible wish that they could study it with modern concepts and the new tools at hand.
Recovery and sequencing of the 1918 influenza virus
Archaevirological search for the 1918 influenza in 1951
One of the co-authors (JVH) was a student at the University of Iowa in 1949, beginning a PhD program in microbiology. In 1950, Dr William Hale (1898–1976), of Brookhaven National Laboratory, visited the University. During a discussion about the 1918 influenza pandemic, he commented ‘someone ought to go to the frozen north to find a victim from 1918 in a permafrost grave’. Immediately after that meeting, Hultin contacted his faculty advisor, Dr Albert McKee (1913–), with a dissertation proposal: to find such a permafrost grave in Alaska. Hultin began by collecting information from Alaska, most importantly from the palaeontologist Otto Geist (1888–1962). With Geist’s help, Hultin contacted several Alaskan missions about their 1918 experiences.
By 1951, all such mission communications had been received. Three sites along the coast of the Seward Peninsula were selected for further study: Nome, Wales, and Brevig Mission. The selection was based on epidemiological evidence indicating high pandemic fatality in the Inuit population. In Nome more than half of the native population had died, in Teller 53%, in Brevig Mission (then called Teller Mission) 90%, in York 100%, and in Wales 55% [81]. Because the mode of burial is of great importance for the preservation of victims, it was most fortunate that gold miners from Nome, skilled in penetrating the permafrost, had been employed by the Territorial government. They had moved from village to village during the winter of 1918–1919, and had managed to bury all of the victims in mass graves 2 m deep.
In June 1951, an expedition consisting of Hultin, McKee, and the team’s renowned pathologist Jack M Layton (1917–), left for Alaska. At Brevig Mission the permafrost conditions were promising, and permission to perform an exhumation was obtained. The team, joined in Alaska by Geist, made rapid progress digging. Reaching a depth of 2 m, a layer of bodies was discovered, placed side by side. Layton opened the rib cages of four bodies, exposing frozen, dark red, expanded lungs. Generous biopsies from eight lungs were obtained and, while still frozen, placed in sterile containers that were then put into thermal jugs and kept frozen with carbon dioxide snow from fire extinguishers. The instruments used were sterilized in boiling water at the graveside; surgical masks were used, as were sterile gloves.
In the microbiology laboratory at the University of Iowa, this material was cultured in embryonated eggs using available containment procedures of the time, including use of face masks, gloves and special pipettes, with all work done under a negative-pressure hood. Five susceptible ferrets received nasal instillations. The ferrets showed no signs of illness. Cultures from the lung material of some of the specimens grew H. influenzae and S. pneumoniae. Histological analyses showed a predominating pattern of acute viral pneumonitis, although some sections showed acute bacterial pneumonias. All of the available specimens were processed but no influenza virus was recovered. Unfortunately, all of the materials from this project were subsequently discarded. As Alfred Crosby stated in his book America’s Forgotten Pandemic, ‘the most direct assault on Spanish influenza had failed’ [82]. He also wrote [83]: ‘It has been the dream of scientists working on influenza for over a half century to somehow obtain specimens of the virus of Spanish influenza, but only something as unlikely as a time capsule could provide them.’
Archaevirological search for the 1918 influenza in 1995
Forty-five years later, in 1995, the search for this time capsule was resumed when co-author JKT began a project to recover RNA fragments of the 1918 influenza virus from formalin-fixed, paraffin-embedded (FFPE) autopsy tissues in the collection of the National Tissue Repository of the Armed Forces Institute of Pathology (AFIP). In the mid-1990s the Molecular Pathology Division of the AFIP had been engaged in developing and optimizing diagnostic molecular assays for neoplastic and infectious diseases that could be applied to FFPE tissues [84]. Projects undertaken simultaneously to characterize novel morbilliviruses from marine mammal epizootics [85], using both poorly preserved unfixed tissues and FFPE necropsy tissues, helped clarify protocols to perform genetic analyses of RNA viruses from sub-optimally preserved tissues.
Thus, by 1995, technical advances were in place that would support efforts to recover genomic material from 1918 influenza victims. The event that persuaded Taubenberger’s team to turn their attention to influenza was the publication of a study describing the genetic basis of British chemist/physicist John Dalton’s colour-blindness using DNA extracted from autopsy tissues from 1844 [86]. This led to the idea of attempting a similar project using some significant archival tissue sample for molecular genetic analysis. Genetic material from the 1918 influenza virus seemed an obvious choice because of its great scientific and historical significance.
A search of the AFIP tissue archives revealed over 100 autopsy cases of 1918 influenza victims. Over 70 had tissue samples associated with them. Review of the case records and histological examination narrowed the likely influenza-RNA-positive cases to 13. Of these, one case was found in 1996 to be positive for influenza A RNA fragments <140 bp in length. Sequence fragments of four gene segments from this case were published in 1997, confirming the H1N1 subtype and demonstrating the lack of a cleavage site mutation in HA [87]. Although the initial results were promising, by 1997 there was concern that unless additional positive case material was found, it might not be possible to determine the entire genomic sequence of the 1918 virus. A second round of screening of AFIP cases in 1997 revealed a second positive case; simultaneously, the Taubenberger laboratory received new case material from a frozen lung sample of a 1918 victim from Brevig Mission, Alaska, contributed by co-author JVH, as described below.
After reading the initial paper in 1997 on the characterization of RNA fragments obtained from the first AFIP 1918 case, Hultin wrote to Taubenberger, detailing the Iowa expedition’s work at Brevig Mission in 1951, and offering to return to Alaska for a second exhumation to secure additional specimens for molecular analysis. At a special meeting of the Brevig City Council, permission to reopen the graves was granted. One of the victims found was an obese female whose body was well preserved. On further excavation, two skeletons were found, one on either side of her. It is likely that the subcutaneous fatty tissue of the obese woman had preserved the internal organs from decomposition during occasional short periods of thawing within the permafrost. Her lungs displayed the gross appearance of those seen in acute viral pneumonitis, expanded and dark red in colour. Samples of frozen lung were placed directly in fixatives, including ethanol and guanidine.
The material from this frozen tissue yielded influenza RNA fragments of a slightly smaller size than those from the two FFPE cases (no greater than 120 bp), but had the advantage of providing more starting material. The HA1 domain of the HA gene was sequenced from all three cases [88], and they differed from each other by only a single nucleotide over 1,200 bases. Because the viruses were therefore probably nearly identical in sequence, it was decided to sequence the remaining seven gene segments from the Alaskan case material.
Determining the complete coding sequence of the 1918 virus took 9 years, including publication of the neuraminidase (NA) segment in 2000 [89], the non-structural segment in 2001 [90], the matrix segment in 2002 [91], the nucleoprotein segment in 2004 [92] and the three polymerase gene segments in 2005 [1]. The search for additional 1918 influenza-RNA-positive cases was also expanded by screening FFPE autopsy tissue blocks from the collection of the Royal London Hospital. Several additional 1918 cases were found, and sequencing of the HA1 domain of HA again revealed extremely high sequence identity between the isolates [93].
Reconstruction of the 1918 virus
The development of reverse genetics technology for influenza viruses in 1999 [94,95] made it possible to perform experiments with viruses containing one or more 1918 influenza genes. This was crucial, because sequence analysis alone offered no clues to the pathogenicity of the 1918 virus. Since 2001, a series of experiments has been conducted in a multicentre, collaborative project to model virulence in vitro, and in animal models using viral constructs containing one or more 1918 genes produced by reverse genetics. The collaborators on this NIAID-funded program project include co-author JKT, Drs Adolfo García-Sastre, Peter Palese and Christopher Basler of Mount Sinai School of Medicine, David Swayne of the US Department of Agriculture (USDA), Terry Tumpey of the US Centers for Disease Control and Prevention (CDC), Michael Katze of the University of Washington, and Ian Wilson of Scripps Research Institute, and their staffs. All work in this collaborative project using 1918 viral constructs has been conducted in BSL3+ containment laboratories at the USDA Southeast Poultry Research Laboratory, or in BSL3+ containment laboratories of the CDC [90,96–98].
Viral constructs bearing at least 1918 HA and NA genes in a background of modern, non-mouse-adapted human H1N1 virus, are all highly pathogenic in mice [97–101]. Furthermore, expression microarray analysis performed on whole lung tissue of mice infected with the reconstructed 1918 virus or viral constructs containing at least the 1918 HA and NA genes showed marked upregulation of murine genes involved in apoptosis, tissue injury, and oxidative damage [100,101]. These findings were unexpected because the viruses with the 1918 HA and NA genes had not been adapted to mice. Control experiments in which mice were infected with modern human viruses produced limited viral replication and little disease, but the lungs of animals infected with the 1918 HA/NA construct showed bronchial and alveolar epithelial necrosis and a marked inflammatory infiltrate, suggesting that the 1918 HA (and possibly the NA) contain virulence factors for mice, but that the full virulent phenotype is only observed with the completely reconstructed virus [98,101].
The viral genotypical basis of this virulence has not yet been mapped, and its relevance for human pathogenesis also remains unclear. The murine pathology, although reminiscent of some of the acute viral pneumonia pathology seen in 1918 autopsy studies [11], is nevertheless distinctive. The roles of the other 1918 proteins, singularly and in combination, are currently unknown. However, the reconstructed all-eight-gene 1918 influenza virus is more virulent than constructs containing fewer 1918 genes, suggesting contributions of each gene segment to virulence [98,101]. Experiments to further map the genetic basis of virulence of the 1918 virus in various animal models are planned. These experiments should help define the viral component of the unusual pathogenicity of the 1918 virus, but cannot address whether specific host factors in 1918 accounted for unique influenza mortality patterns, such as increased fatality in 20–40 year olds and possible protection in the elderly [11].
Viral sequence data now suggest that the entire 1918 virus was novel to humans in, or shortly before, 1918, and that it was not likely to have been a reassortant virus such as those that caused the 1957 and 1968 pandemics [102]. Rather, the 1918 virus is an avian-influenza-like virus that appears to have been derived in toto from an unknown source [1,92,103] because its eight genome segments differ from contemporary avian influenza genes, especially at synonymous sites. Influenza virus gene sequences from a number of fixed specimens of wild birds collected circa 1918 showed little difference from avian viruses isolated today and consequently did not suggest these birds were the source [104,105]. These findings also suggest that avian viruses undergo little directed evolution in their natural hosts even over long periods.
In collaboration with Dr John Oxford, a new project to expand knowledge of human influenza virus circulation before 1918 was initiated in 2004, using additional samples from the post mortem tissue archives of the Royal London hospital. Recently, several pre-1918-human-influenza-A-RNA-positive cases have been identified and initial genetic characterization is ongoing (unpublished). The goal of this project is twofold: (i) to determine what subtype(s) circulated in humans before 1918, and (ii) to determine whether any previously human-adapted influenza gene segments were retained in the 1918 pandemic virus. Concurrently, autopsy specimens from influenza cases from the 1920s and 1930s are also being examined to characterize the early evolution of human H1N1 viruses prior to the first H1N1 isolations in the 1930s; several influenza A RNA-positive cases from the 1920s have already been identified and are now being studied. If there are specific genotypic traits that gave the 1918 virus its particular virulence for young adults, comparing it experimentally with less pathogenic descendant viruses from the early 1920s might be especially informative.
Conclusions and future work
The current projects to understand the origin of the 1918 influenza pandemic virus and its virulence characteristics rest on a solid foundation of influenza virology and epidemiology developed over the last century. It is hoped that additional insights into the mechanisms of viral host adaptation and the mechanisms of how influenza viruses cause disease in their human and experimental hosts will come from future work with the reconstructed 1918 virus. Looking backward in time, but also looking forward into the future, we can see that science stands in the middle of a long and continuing line of effort to comprehend history’s most devastating human disease. We must also be aware that revealing the biology of a pandemic that occurred nearly 90 years ago is not just a historical exercise. It may well help us prepare for, and even prevent, the emergence of new pandemics in the 21st century and beyond.
Acknowledgments
For assistance in obtaining historical manuscripts we are grateful to Steven Greenberg, PhD and the staff of the History of Medicine Division, to Deirdre Clarkin and the staff of the Public Services Division, National Library of Medicine, and to Betty Murgolo and the staff of the National Institutes of Health Library.
References
- 1.Taubenberger JK, Reid AH, Lourens RM, Wang R, Jin G, Fanning TG. Characterization of the 1918 influenza virus polymerase genes. Nature. 2005;437:889–893. doi: 10.1038/nature04230. [DOI] [PubMed] [Google Scholar]
- 2.Webster RG, Peiris M, Chen H, Guan Y. H5N1 outbreaks and enzootic influenza. Emerg Infect Dis. 2006;12:3–8. doi: 10.3201/eid1201.051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snow J. On the Mode of Communication of Cholera. 2. London: John Churchill; 1855. much enlarged. [Google Scholar]
- 4.Rahts Die Influenza-epidemie des Winters 1893/94 im Deutschen Reiche [The influenza epidemic of the Winter of 1893/94 in the German Empire] Arbeiten aus dem Kaiserlichen Gesundheitsamte. 1896;12:423–447. [Google Scholar]
- 5.Kendal AP, Minuse E, Maassab HF, Hennessy AV, Davenport FM. Influenza neuraminidase antibody patterns of man. Am J Epidemiol. 1973;98:96–103. doi: 10.1093/oxfordjournals.aje.a121543. [DOI] [PubMed] [Google Scholar]
- 6.Koch R. Die Aetiologie der Milzbrand-Krankheit, begründet auf die Entwicklungsgeschichte der Bacillus Anthracis [The aetiology of the illness of anthrax, based on the developing history of Bacillus anthracis] Beiträge zur Biologie der Pflanzen (Breslau [Wroclaw]) 1876;2:277–310. [Google Scholar]
- 7.Pfeiffer R. Aus dem Institut für Infektionskrankheiten. II. Vorläufige Mittheilungen über die Erreger der Influenza [From the Institute for Infectious Diseases. II. Provisional communication on the cause of influenza] Deutsche medicinische Wochenschrift. 1892;18:28. [Google Scholar]
- 8.Kitasato S. Aus dem Institut für Infektionskrankheiten. III. Ueber den Influenzabacillus und sein Culturverfahren [From the Institute for Infectious Diseases. III. On the influenza bacillus and its method of cultivation] Deutsche medicinische Wochenschrift. 1892;18:28. [Google Scholar]
- 9.Pfeiffer R. Die Aetiologie der Influenza [The aetiology of influenza] Zeitschrift für Hygiene und Infektionskrankheiten; medizinische Mikrobiologie, Immunologie und Virologie. 1893;13:357–385. [Google Scholar]
- 10.Olitsky P, Gates F. Experimental study of the nasopharyngeal secretions from influenza patients. J Am Med Assoc. 1920;74:1497–1499. [Google Scholar]
- 11.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeiffer R, Kolle W. Ueber die specifische Immunitätsreaction der Typhusbacillen [On the specific immune reaction to the typhus bacillus] Zeitschrift für Hygiene und Infektionskrankheiten. 1895–1896;21:203–246. [Google Scholar]
- 13.Pfeiffer R, Kolle W. Experimentelle Untersuchungen zur Frage de Schutzimpfung des Menschen gegen Typhus abdominalis [Experimental investigations on the question of human immunization against Typhus abdominalis] Deutsche medizinische Wochenschrift. 1896;22:735–737. [Google Scholar]
- 14.Rietschel E, Cavaillon J-M. Richard Pfeiffer and Alexandre Besredka: creators of the concept of endotoxin and anti-endotoxin. Microbes Infect. 2003;5:1407–1414. doi: 10.1016/j.micinf.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Kitasato S. Ueber den Tetanusbacillus [On the tetanus bacillus] Zeitschrift für Hygiene und Infektionskrankheiten; medizinische Mikrobiologie, Immunologie und Virologie. 1889;7:225–234. [Google Scholar]
- 16.von Behring E, Kitasato S. Ueber das Zustandekommen der Diptherie-Immunität und der Tetanus-Immunität bei Thieren [On the development of diphtheria immunity and tetanus immunity in animals] Deutsche medizinische Wochenschrift. 1890;16:1113–1114. [Google Scholar]
- 17.Kitasato S. The bacillus of bubonic plague. Lancet. 1894;2:428–430. [Google Scholar]
- 18.Ivanovski D. Ueber die Mosaikkrankheit der Tabakspflanze [On the mosaic disease of tobacco plants] Sel’skoe khozyaistvo i lêsovodstvo. 1892;35:67–70. [Google Scholar]
- 19.Beijerinck M. Over een Contagium vivum fluidum als oorzaak van de Vlekziekte der Tabaksbladen [Concerning a contagium vivum fluidum as cause of the spot disease of tobacco leaves] Koninklijke Akademie van Wetenschappen te Amsterdam. Verslag van de gewone vergaderingen der wis- en natuurkundige Afdeeling, van 28 Mei 1898 tot 22 April 1899. 1898–1899;7:229–235. [Google Scholar]
- 20.Wilkinson L. The development of the virus concept as reflected in corpora of studies on individual pathogens. 1. Beginnings at the turn of the century. Med Hist. 1974;18:211–221. doi: 10.1017/s0025727300019578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loeffler F, Frosch P. Summarischer Bericht über die Ergebnisse der Untersuchungen der Kommission zur Erforschung der Maul- und Klauenseuche bei dem Institut für Infektionskrankheiten in Berlin [Summary report on the outcomes of the investigation of the commission for researching foot and mouth disease of the Institute for Infectious Diseases in Berlin] Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten 1 Abteilung: medizinische-hygienische Bakteriologie und tierische Parasitenkunde. 1897;22:257–259. [Google Scholar]
- 22.Loeffler F, Frosch P. Bericht der Kommission zur Erforschung der Maul- und Klauenseuche bei dem Institut für Infektionskrankheiten in Berlin [Report of the commission for researching foot and mouth disease of the Institute for Infectious Diseases in Berlin]. Centralblatt für Bakteriologie und Infektionskrankheiten. 2 naturwissenschaftenliche Abteilung: Allgemeine, land-wirtschaftliche-technologische Bakteriologie. Gärungsphysiologie und Pflanzenphysiologie. 1898;23:371–391. [Google Scholar]
- 23.Nocard E, Roux P. Le microbe de la peripneumonie [The microbe of peripneumonia] Annales de l’Institut Pasteur. 1889;12:240–262. [Google Scholar]
- 24.Sanarelli G. Das myxomatogene Virus [The myxomatogenous virus] Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten 1 Abteilung: medizinische-hygienische Bakteriologie und tierische Parasitenkunde. 1898;23:865–873. [Google Scholar]
- 25.M’fadyean J. African horse-sickness. J Comp Path Ther. 1900;13:1–20. [Google Scholar]
- 26.Roux É. Sur les microbes dites “invisibles” [On the microbes known as ‘invisible’] Bulletin de l’Institut Pasteur. 1903;1:7–12. 49–56. [Google Scholar]
- 27.Remlinger P. Les microbes filtrants [The filter-passing microbes] Bulletin de l’Institut Pasteur. 1906;4:337–345. 385–392. [Google Scholar]
- 28.Foster G. The etiology of common colds. The probable role of a filtrable virus as the causative factor: with experiments on the cultivation of a minute micro-organism from the nasal secretion filtrates. J Infect Dis. 1917;21:451–474. [Google Scholar]
- 29.Fleming G. Animal Plagues: History, Nature and Prevention. Vol. 1. London: Baillière, Tindall & Cox; 1871. [Google Scholar]
- 30.Chun J. Influenza including its infection among pigs. Nat Med J. 1919–1920;5:34–44. [Google Scholar]
- 31.Koen J. A practical method for field diagnosis of swine diseases. Am J Vet Med. 1919;14:468–470. [Google Scholar]
- 32.Perroncito E. Epizoozia tifoide nei gallinacei [Epizootic of typhoid in gallinaceous birds] Ann Accad Agric Torino. 1878;21:87–126. [Google Scholar]
- 33.Lode A, Gruber F. Bakteriologische Studien über die Aetiologie einer epidemischen Erkrankung der Hühner in Tirol (1901) [Bacteriological studies on the aetiology of epidemic illness of chickens in Tirol (1901)] Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten 1 Abteilung: medizinische-hygienische Bakteriologie und tierische Parasitenkunde. 1901;30:593–604. [Google Scholar]
- 34.Centanni E. Die Vogelpest. Beitrag zu dem durch Kerzen filtrierbaren Virus [Fowl plague. Report on the candle filterable virus] Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten 1 Abteilung: medizinische-hygienische Bakteriologie und tierische Parasitenkunde. 1902;31:145–152. [Google Scholar]
- 35.Maggiora A, Valenti G. Ueber eine Seuche von exsudtivem Typhys bei Hühnern. I. Mittheilung [Regarding an epidemic of exudative typhus in chickens. Part I] Zeitschrift für Hygiene und Infektionskrankheiten; medizinische Mikrobiologie, Immunologie und Virologie. 1903;42:185–243. [Google Scholar]
- 36.Schäfer W. Vergleichende sero-immunologische Untersuchungen über die Viren der Influenza und klassichen Geflügelpest [Comparative sero-immunological investigations on the viruses of influenza and classical fowl plague] Zeitschrift für Naturforschung. 1955;10b:81–91. [Google Scholar]
- 37.Pereira HG, Tumová B, Law VG. Avian influenza A viruses. Bulletin of the World Health Organization. 1965;32:855–860. [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira HG, Tumová B, Webster RG. Antigenic relationship between influenza A viruses of human and avian origins. Nature. 1967;215:982–983. doi: 10.1038/215982a0. [DOI] [PubMed] [Google Scholar]
- 39.Slemons RD, Johnson DC, Osborn JS, Hayes F. Type A influenza viruses isolated from wild free-flying ducks in California. Avian Diseases. 1974;18:119–125. [PubMed] [Google Scholar]
- 40.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiological Reviews. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeiffer R. Das Influenzaproblem [The influenza problem] Ergebnisse der Hygiene, Bakteriologie, Immunitätsforschung und experimentellen Therapie. 1922;5:1–18. [Google Scholar]
- 42.Pfeiffer R. Neuere Forschungen zur Klärung der Influenzaätiologie [New research to clarify the aetiology of influenza] Deutsche medizinische Wochenschrift. 1925;51:10–13. [Google Scholar]
- 43.LeCount E. The pathologic anatomy of influenzal bron-chopneumonia. J Am Med Assoc. 1919;72:650–652. [Google Scholar]
- 44.Wolbach S. Comments on the pathology and bacteriology of fatal influenza cases, as observed at Camp Devens, Mass. Johns Hopkins Hospital Bulletin. 1919;30:104–109. [Google Scholar]
- 45.Winternitz M, Wason I, McNamara F. The Pathology of Influenza. Vol. 61. New Haven: Yale University Press; 1920. [Google Scholar]
- 46.Nicolle C, Lebailly C. Quelques notions expérimentales sur le virus de la grippe [Certain experimental ideas about the infectious agent of influenza] Comptes rendus de l’Académie des Sciences. 1918;167:607–610. [Google Scholar]
- 47.Yamanouchi T, Sakakami K, Iwashima S. The infecting agent in influenza. an experimental research Lancet. 1919;1:971. [Google Scholar]
- 48.Selter H. Zur Aetiologie der Influenza [On the aetiology of influenza] Deutsche medizinische Wochenschrift. 1918;44:932–933. [Google Scholar]
- 49.Keegan J. The prevailing pandemic of influenza. J Am Med Assoc. 1918;71:1051–1055. [Google Scholar]
- 50.Dujarric de la Rivière M. La grippe est-elle une maladie à virus filtrant? [Is influenza a disease of filter-passing microbes?] Comptes rendus de l’Académie des Sciences. 1918;167:606–607. [Google Scholar]
- 51.Gibson H, Bowman F, Connor J. A filtrable virus as the cause of the early stage of the present epidemic of influenza. Brit Med J. 1918;4:645–646. doi: 10.1136/bmj.2.3024.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Angerer Ein filtrierbaren Erreger der Grippe [A filterable cause of influenza] Münchener medizinische Wochenschrift. 1918;65:1280. [Google Scholar]
- 53.Binder A. Studien zur Aetiologie der Influenza. I. Zur mikroskopischen Diagnose der Influenza [Studies on the aetiology of influenza. I. On the microscopic diagnosis of influenza] Münchener medizinische Wochenschrift. 1918;65:1397–1398. [Google Scholar]
- 54.Prell H., II Die Erreger der Influenza [The causes of influenza] Münchener medizinische Wochenschrift. 1918;65:1398–1399. [Google Scholar]
- 55.Leschke E. Untersuchungen zur Aetiologie der Grippe [Studies on the aetiology of influenza] Berliner klinische Wochenschrift. 1919;56:11–12. [Google Scholar]
- 56.Bradford J, Bashford E, Wilson J. Preliminary report on the presence of a “filter passing” virus in certain diseases, with especial reference to trench fever, influenza, and nephritis. Brit Med J. 1919;1:127–128. doi: 10.1136/bmj.1.3031.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cummins S. Cultivation of a filter–passing organism in influenza. Lancet. 1919;1:528. [Google Scholar]
- 58.Lister F. A filter-passing micro-organism associated with influenza. South African Medical Record. 1922;20:434–436. [Google Scholar]
- 59.Arkwright J. A criticism of certain recent claims to have discovered and cultivated the filter-passing virus of trench fever and of influenza. Brit Med J. 1919;2:233–236. doi: 10.1136/bmj.2.3060.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fejes L. Die Aetiologie der Influenza [The aetiology of influenza] Deutsche medizinische Wochenschrift. 1919;45:653–654. [Google Scholar]
- 61.Maitland H, Cowan M, Detweiler H. The aetiology of epidemic influenza: experiments in search of a filter-passing virus. Brit J Exp Path. 1920;1:263–281. [Google Scholar]
- 62.Hall M. A study of the lesions produced by filtrates of influenza sputum. Arch Int Med. 1920;26:612–629. [Google Scholar]
- 63.Loewe L, Zeman F. Cultivation of a filtrable organism from the nasopharyngeal washings in influenza. J Am Med Assoc. 1921;76:986–988. [Google Scholar]
- 64.Abe T, Ishikawa T, Nishibe M, Nakata I, Tenjin S. Influenza Research Committee, The Government Institute for Infectious Diseases. Tokyo: Tokyo Imperial University; 1922. An experimental study of filtering property of influenza virus upon certain animals. Studies on Influenza From the Influenza Research Committee of the Institute, December 28, 1922; pp. 29–33. [Google Scholar]
- 65.Gordon M. The filter passer of influenza. The properties of filtrable viruses. J Roy Army Med Corps. 1922;39:1–13. [Google Scholar]
- 66.Woodcock H. Are the active principles of filter-passing and “ultramicroscopic” viruses living organisms or enzymes? J Roy Army Med Corps. 1922;39:244–260. [Google Scholar]
- 67.Detweiler H, Hodge W. An attempt to isolate a filter-passing virus in epidemic influenza. J Exp Med. 1924;39:43–50. doi: 10.1084/jem.39.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boëz L. Les microbes filtrables des voies respiratoires dans l’influenza et le coryza aigu [Filterable microbes of the respiratory tract in influenza and acute coryza] Annales de l’Institut Pasteur. 1925;39:833–854. [Google Scholar]
- 69.Rosenau MJ, Keegen WJ, Goldberger J, Lake GC. Experiments upon volunteers to determine the cause and mode of spread of influenza, Boston, November and December 1918. Treasury Department, United States Public Health Service Hygienic Laboratory Bulletin. 1921;123:5–41. [Google Scholar]
- 70.Shope R. Swine influenza. I. Experimental transmission and pathology. J Exp Med. 1931;54:349–359. doi: 10.1084/jem.54.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewis P, Shope R. Swine influenza. II. A hemophilic bacillus from the respiratory tract of infected swine. J Exp Med. 1931;54:361–371. doi: 10.1084/jem.54.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shope R. Swine influenza. III. Filtration experiments and etiology. J Exp Med. 1931;54:373–385. doi: 10.1084/jem.54.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanegae Y, Sugita S, Shortridge KF, Yoshioka Y, Nerome K. Origin and evolutionary pathways of the H1 hemagglutinin gene of avian, swine and human influenza viruses: cocirculation of two distinct lineages of swine virus. Arch Virol. 1994;134:17–28. doi: 10.1007/BF01379103. [DOI] [PubMed] [Google Scholar]
- 74.Taubenberger JK, Reid AH, Janczewski TA, Fanning TG. Integrating historical, clinical and molecular genetic data in order to explain the origin and virulence of the 1918 Spanish influenza virus. Philos Trans R Soc Lond B Biol Sci. 2001;356:1829–1839. doi: 10.1098/rstb.2001.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakajima K, Desselberger U, Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978;274:334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- 76.Dochez A, Mills K, Kneeland Y. Studies of the etiology of influenza. Proc Soc Exp Biol Med. 1934–1935;30:1017–1022. [Google Scholar]
- 77.Smith W, Andrewes C, Laidlaw P. A virus obtained from influenza patients. Lancet. 1933;2:66–68. [Google Scholar]
- 78.Francis T, Magill T. The antibody response of human subjects vaccinated with the virus of human influenza. J Exp Med. 1937;65:251–259. doi: 10.1084/jem.65.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smorodintsev A, Shishkina O. Experimental analysis of immune sera. Arkhiv biologicheskikh nauk. 1938;52:132–154. [Google Scholar]
- 80.Salk J, Lavin G, Francis T. The antigenic potency of epidemic influenza virus following inactivation by ultraviolet radiation. J Exp Med. 1940;72:729–745. doi: 10.1084/jem.72.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crosby A. America’s Forgotten Pandemic. 2. New York: Cambridge University Press; 2003. p. 337. [Google Scholar]
- 82.Crosby A. America’s Forgotten Pandemic. 2. New York: Cambridge University Press; 2003. p. 306. [Google Scholar]
- 83.Crosby A. America’s Forgotten Pandemic. 2. New York: Cambridge University Press; 2003. p. 295. [Google Scholar]
- 84.Krafft AE, Duncan BW, Bijwaard KE, Taubenberger JK, Lichy JH. Optimization of the isolation and amplification of RNA from formalin-fixed, paraffin-embedded tissue: The Armed Forces Institute of Pathology experience and literature review. Mol Diagn. 1997;2:217–230. doi: 10.1054/MODI00200217. [DOI] [PubMed] [Google Scholar]
- 85.Taubenberger JK, Tsai M, Krafft AE, et al. Two morbilliviruses implicated in bottlenose dolphin epizootics. Emerg Infect Dis. 1996;2:213–216. doi: 10.3201/eid0203.960308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hunt DM, Dulai KS, Bowmaker JK, Mollon JD. The chemistry of John Dalton’s color blindness. Science. 1995;267:984–988. doi: 10.1126/science.7863342. [DOI] [PubMed] [Google Scholar]
- 87.Taubenberger JK, Reid AH, Krafft AE, Bijwaard KE, Fanning TG. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science. 1997;275:1793–1796. doi: 10.1126/science.275.5307.1793. [DOI] [PubMed] [Google Scholar]
- 88.Reid AH, Fanning TG, Hultin JV, Taubenberger JK. Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proc Natl Acad Sci U S A. 1999;96:1651–1656. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reid AH, Fanning TG, Janczewski TA, Taubenberger JK. Characterization of the 1918 “Spanish” influenza virus neuraminidase gene. Proc Natl Acad Sci U S A. 2000;97:6785–6790. doi: 10.1073/pnas.100140097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Basler CF, Reid AH, Dybing JK, et al. Sequence of the 1918 pandemic influenza virus nonstructural gene (NS) segment and characterization of recombinant viruses bearing the 1918 NS genes. Proc Natl Acad Sci U S A. 2001;98:2746–2751. doi: 10.1073/pnas.031575198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reid AH, Fanning TG, Janczewski TA, McCall S, Taubenberger JK. Characterization of the 1918 “Spanish” influenza virus matrix gene segment. J Virol. 2002;76:10717–10723. doi: 10.1128/JVI.76.21.10717-10723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Reid AH, Fanning TG, Janczewski TA, Lourens RM, Taubenberger JK. Novel origin of the 1918 pandemic influenza virus nucleoprotein gene. J Virol. 2004;78:12462–12470. doi: 10.1128/JVI.78.22.12462-12470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reid AH, Janczewski TA, Lourens RM, et al. 1918 influenza pandemic caused by highly conserved viruses with two receptor-binding variants. Emerg Infect Dis. 2003;9:1249–1253. doi: 10.3201/eid0910.020789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neumann G, Watanabe T, Ito H, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fodor E, Devenish L, Engelhardt OG, Palese P, Brownlee GG, Garcia-Sastre A. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–9682. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tumpey TM, Garcia-Sastre A, Mikulasova A, et al. Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus. Proc Natl Acad Sci U S A. 2002;99:13849–13854. doi: 10.1073/pnas.212519699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Basler CF. Pathogenicity and immuno-genicity of influenza viruses with genes from the 1918 pandemic virus. Proc Natl Acad Sci U S A. 2004;101:3166–3171. doi: 10.1073/pnas.0308391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tumpey TM, Basler CF, Aguilar PV, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 99.Tumpey TM, Garcia-Sastre A, Taubenberger JK, et al. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kash JC, Basler CF, Garcia-Sastre A, et al. Global host immune response: pathogenesis and transcriptional profiling of type A influenza viruses expressing the hemagglutinin and neuraminidase genes from the 1918 pandemic virus. J Virol. 2004;78:9499–9511. doi: 10.1128/JVI.78.17.9499-9511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kash JC, Tumpey TM, Proll SC, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rabadan R, Levine AJ, Robins H. Comparison of avian and human influenza A viruses reveals a mutational bias on the viral genomes. J Virol. 2006;80:11887–11891. doi: 10.1128/JVI.01414-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol. 2004;2:909–914. doi: 10.1038/nrmicro1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fanning TG, Slemons RD, Reid AH, Janczewski TA, Dean J, Taubenberger JK. 1917 avian influenza virus sequences suggest that the 1918 pandemic virus did not acquire its hemagglutinin directly from birds. J Virol. 2002;76:7860–7862. doi: 10.1128/JVI.76.15.7860-7862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reid AH, Fanning TG, Slemons RD, Janczewski TA, Dean J, Taubenberger JK. Relationship of pre-1918 avian influenza HA and NP sequences to subsequent avian influenza strains. Avian Dis. 2003;47 (Suppl 3):921–925. doi: 10.1637/0005-2086-47.s3.921. [DOI] [PubMed] [Google Scholar]


