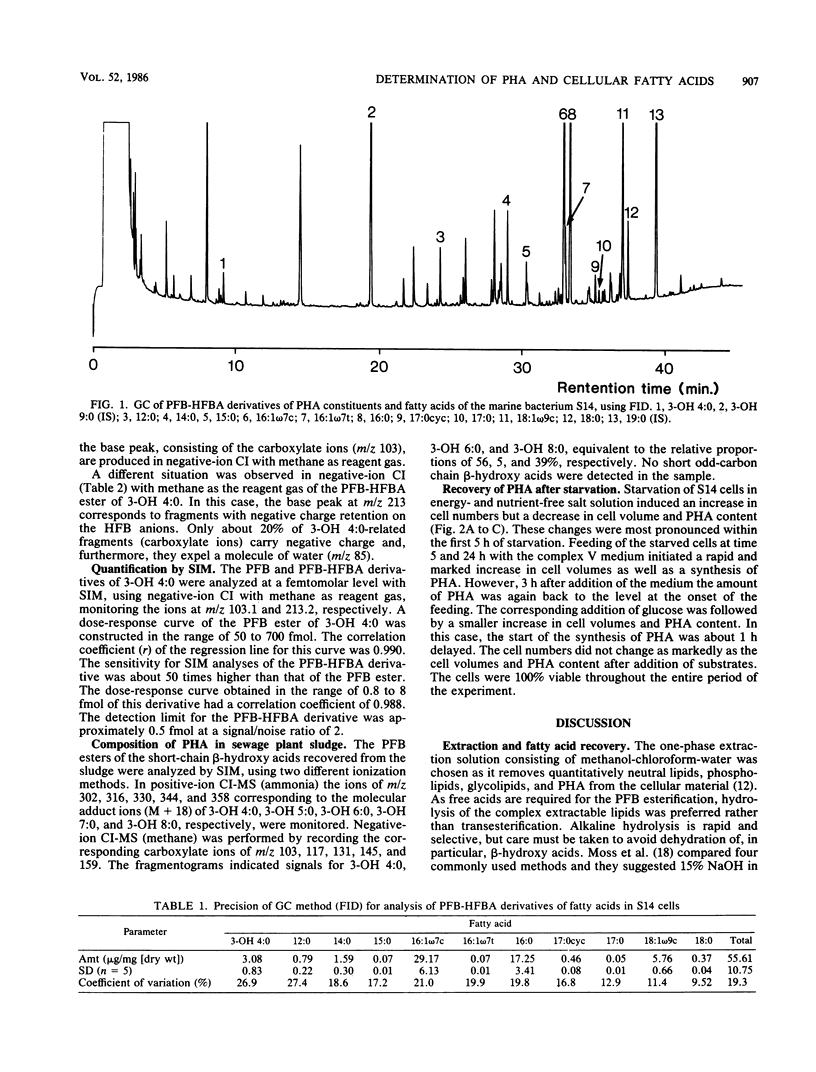
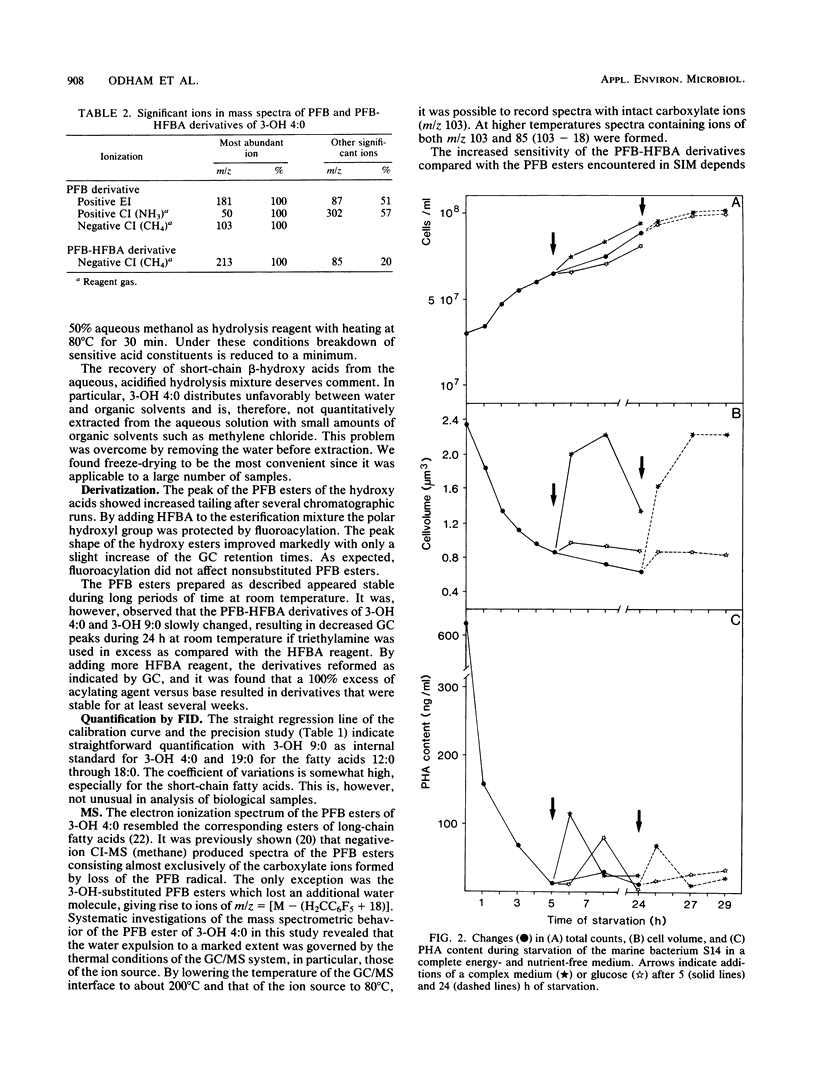
Abstract
Extraction of lipids from bacterial cells or sewage sludge samples followed by simple and rapid extraction procedures and room temperature esterification with pentafluorobenzylbromide allowed combined determinations of poly-β-hydroxyalkanoate constituents and fatty acids. Capillary gas chromatography and flame ionization or mass spectrometric detection was used. Flame ionization permitted determination with a coefficient of variation ranging from 10 to 27% at the picomolar level, whereas quantitative chemical ionization mass spectrometry afforded sensitivities for poly-β-hydroxyalkanoate constituuents in the attomolar range. The latter technique suggests the possibility of measuring such components in bacterial assemblies with as few as 102 cells. With the described technique using flame ionization detection, it was possible to study the rapid formation of poly-β-hydroxyalkanoate during feeding of a starved marine bacterium isolate with a complex medium or glucose and correlate the findings to changes in cell volumes. Mass spectrometric detection of short β-hydroxy acids in activated sewage sludge revealed the presence of 3-hydroxybutyric, 3-hydroxyhexanoic, and 3-hydroxyoctanoic acids in the relative proportions of 56, 5 and 39%, respectively. No odd-chain β-hydroxy acids were found.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Baxter M., Sieburth J. M. Metabolic and ultrastructural response to glucose of two eurytrophic bacteria isolated from seawater at different enriching concentrations. Appl Environ Microbiol. 1984 Jan;47(1):31–38. doi: 10.1128/aem.47.1.31-38.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbie R. J., White D. C. Characterization of benthic microbial community structure by high-resolution gas chromatography of Fatty Acid methyl esters. Appl Environ Microbiol. 1980 Jun;39(6):1212–1222. doi: 10.1128/aem.39.6.1212-1222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck B., Hermansson M., Kjelleberg S., Norkrans B. The hydrophobicity of bacteria - an important factor in their initial adhesion at the air-water interface. Arch Microbiol. 1981 Jan;128(3):267–270. doi: 10.1007/BF00422527. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Findlay R. H., White D. C. Polymeric Beta-Hydroxyalkanoates from Environmental Samples and Bacillus megaterium. Appl Environ Microbiol. 1983 Jan;45(1):71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyllenhaal O., Brötell H., Hartvig P. Determination of free fatty acids as pentafluorobenzyl esters by electron capture gas chromatography. J Chromatogr. 1976 Dec 22;129:295–302. doi: 10.1016/s0021-9673(00)87787-0. [DOI] [PubMed] [Google Scholar]
- Humphrey B., Kjelleberg S., Marshall K. C. Responses of marine bacteria under starvation conditions at a solid-water interface. Appl Environ Microbiol. 1983 Jan;45(1):43–47. doi: 10.1128/aem.45.1.43-47.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. D., White D. C., Taylor C. W. Use of lipid composition and metabolism to examine structure and activity of estuarine detrital microflora. Appl Environ Microbiol. 1977 May;33(5):1177–1183. doi: 10.1128/aem.33.5.1177-1183.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Veldhuis C., Stegeman V., Veenhuis M. Selective advantage of a Spirillum sp. in a carbon-limited environment. Accumulation of poly-beta-hydroxybutyric acid and its role in starvation. J Gen Microbiol. 1979 Jun;112(2):349–355. doi: 10.1099/00221287-112-2-349. [DOI] [PubMed] [Google Scholar]
- Moskowitz G. J., Merrick J. M. Metabolism of poly-beta-hydroxybutyrate. II. Enzymatic synthesis of D-(-)-beta hydroxybutyryl coenzyme A by an enoyl hydrase from Rhodospirillum rubrum. Biochemistry. 1969 Jul;8(7):2748–2755. doi: 10.1021/bi00835a009. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Lambert M. A., Merwin W. H. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974 Jul;28(1):80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickels J. S., King J. D., White D. C. Poly-beta-Hydroxybutyrate Accumulation as a Measure of Unbalanced Growth of the Estuarine Detrital Microbiota. Appl Environ Microbiol. 1979 Mar;37(3):459–465. doi: 10.1128/aem.37.3.459-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


