Abstract
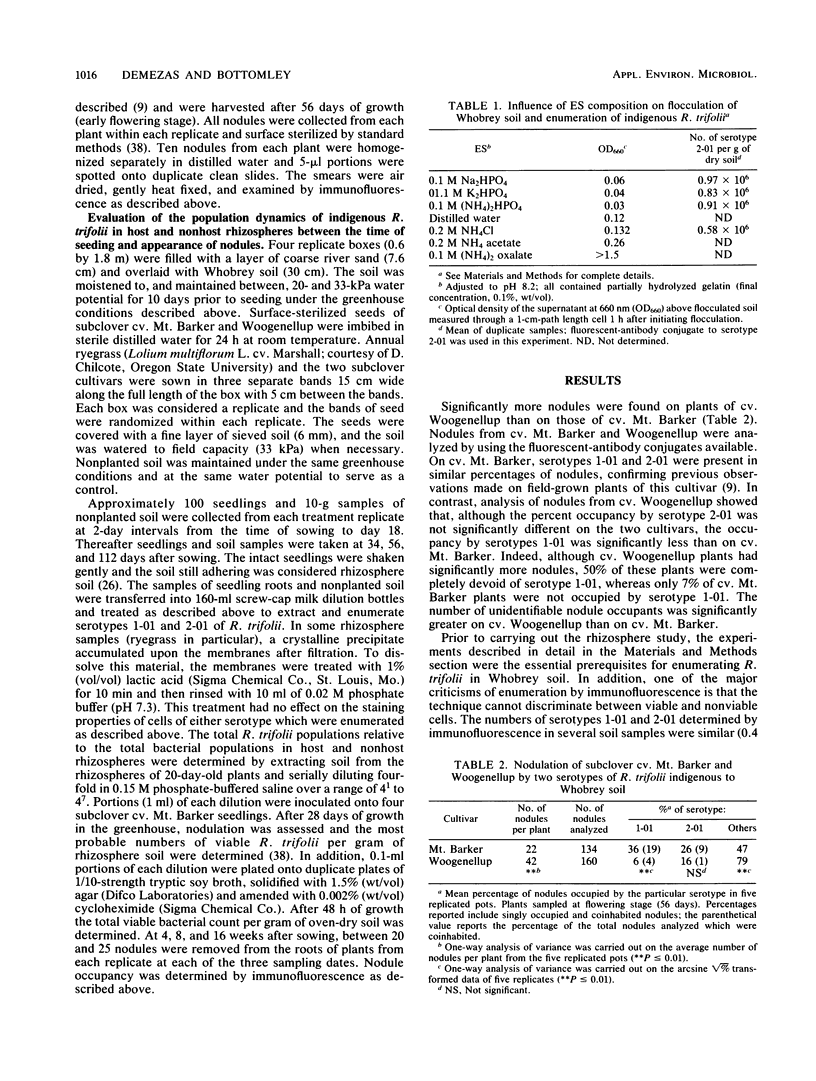
Indigenous serotype 1-01 of Rhizobium trifolii occupied significantly fewer nodules (6%) on plants of soil-grown noninoculated subterranean clover (Trifolium subterraneum L.) cv. Woogenellup than on cv. Mt. Barker (36%) sampled at the flowering stage of growth. Occupancy by indigenous serotype 2-01, was not significantly different on the two cultivars (16 and 26%). Serotype-specific, fluorescent-antibody conjugates were synthesized and used to enumerate the indigenous serotypes in host (clovers) and nonhost (annual rye-grass, Lolium multiflorum L.) rhizospheres and in nonplanted soil. The form and concentration of Ca2+ in the flocculating mixture and the presence of phosphate anions in the extracting solution were both critical for enumerating R. trifolii in Whobrey soil. The two serotypes were present in similar numbers in nonplanted soil (ca. 106 per g of soil) and each represented ca. 10% of the total R. trifolii population. Although host rhizospheres did not preferentially stimulate either serotype, the mean population densities of serotype 2-01 were significantly greater (P = 0.05) than those of serotype 1-01 in clover rhizospheres on 8 of 14 samplings made between the time of seeding and the appearance of nodules (day 12). In this experiment, and in contrast to our earlier findings, serotype 1-01 occupied significantly fewer (P ≤ 0.05) of the nodules (7 to 16%) on both cultivars than serotype 2-01 (51%) when sampled at 4 weeks. Differences between cultivars became apparent as the plants matured. There was a threefold increase (7 to 21%) in nodules occupied by serotype 1-01 on cv. Mt. Barker between 4 and 16 weeks. This was accompanied by increases in nodules coinhabited by both nonidentifiable occupants and either serotype 1-01 (0 to 20%) or 2-01 (11 to 51%). No increases in either of these parameters were observed on cv. Woogenellup.
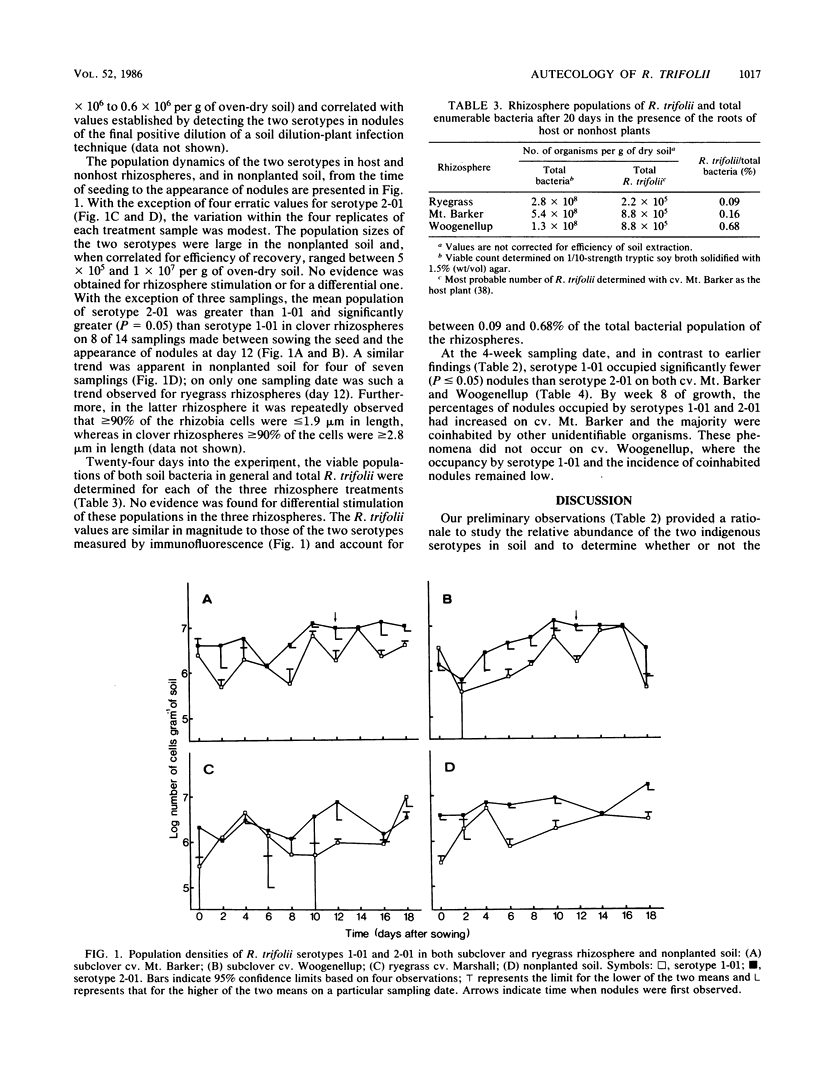
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohlool B. B., Schmidt E. L. Nonspecific staining: its control in immunofluorescence examination of soil. Science. 1968 Nov 29;162(3857):1012–1014. doi: 10.1126/science.162.3857.1012. [DOI] [PubMed] [Google Scholar]
- Demezas D. H., Bottomley P. J. Interstrain Competition between Representatives of Indigenous Serotypes of Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1020–1025. doi: 10.1128/aem.52.5.1020-1025.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabak E. M., Urbano M. R., Dazzo F. B. Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind trifoliin A, a white clover lectin. J Bacteriol. 1981 Nov;148(2):697–711. doi: 10.1128/jb.148.2.697-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A. W., Beringer J. E. Identification of the rhizobium strains in pea root nodules using genetic markers. J Gen Microbiol. 1975 Apr;87(2):343–350. doi: 10.1099/00221287-87-2-343. [DOI] [PubMed] [Google Scholar]
- Kingsley M. T., Bohlool B. B. Release of Rhizobium spp. from Tropical Soils and Recovery for Immunofluorescence Enumeration. Appl Environ Microbiol. 1981 Aug;42(2):241–248. doi: 10.1128/aem.42.2.241-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May S. N., Bohlool B. B. Competition Among Rhizobium leguminosarum Strains for Nodulation of Lentils (Lens esculenta). Appl Environ Microbiol. 1983 Mar;45(3):960–965. doi: 10.1128/aem.45.3.960-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes V. G., Schmidt E. L. Population Densities of Rhizobium japonicum Strain 123 Estimated Directly in Soil and Rhizospheres. Appl Environ Microbiol. 1979 May;37(5):854–858. doi: 10.1128/aem.37.5.854-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert F. M., Schmidt E. L. Population Changes and Persistence of Rhizobium phaseoli in Soil and Rhizospheres. Appl Environ Microbiol. 1983 Feb;45(2):550–556. doi: 10.1128/aem.45.2.550-556.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINCENT J. M., WATERS L. M. The influence of the host on competition amongst clover root-nodule bacteria. J Gen Microbiol. 1953 Dec;9(3):357–370. doi: 10.1099/00221287-9-3-357. [DOI] [PubMed] [Google Scholar]
- Wollum A. G., Miller R. H. Density Centrifugation Method for Recovering Rhizobium spp. from Soil for Fluorescent-Antibody Studies. Appl Environ Microbiol. 1980 Feb;39(2):466–469. doi: 10.1128/aem.39.2.466-469.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



