Abstract
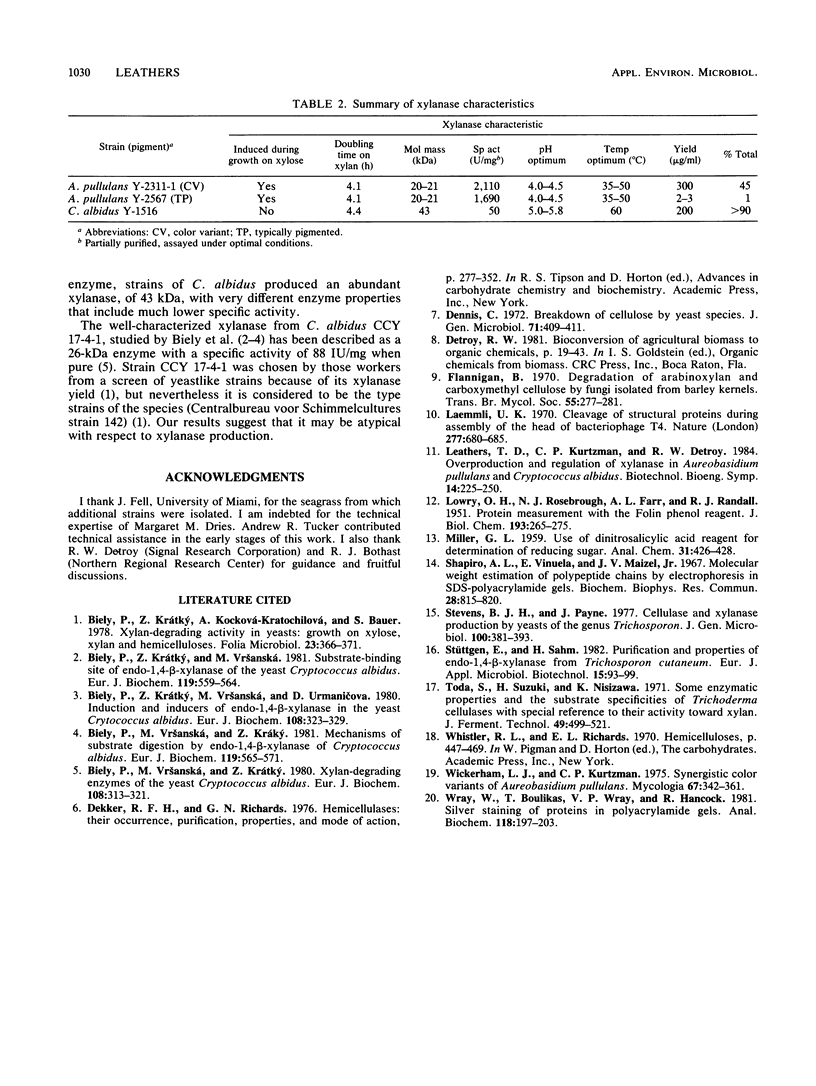
Xylanase activity from naturally occurring color variants of Aureobasidium pullulans was associated with extracellular monomeric proteins of 20 to 21 kilodaltons. Xylanase represented nearly half the total extracellular protein, with a yield of up to 0.3 g of xylanase per liter. The specific activity of partially purified xylanase exceeded 2,000 IU/mg. Xylanase from typically pigmented strains appeared similar to that from color variants with respect to molecular weight, pH and temperature optima, and specific activity of purified (but not crude) enzyme. However, xylanase from typical strains made up only about 1.0% of total extracellular protein. Xylanase from strains of Cryptococcus albidus was associated with abundant proteins of about 43 kilodaltons and showed much lower specific activity.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biely P., Krátký Z., Kocková-Kratochvílová A., Bauer S. Xylan-degrading activity in yeasts: growth on xylose, xylan and hemicelluloses. Folia Microbiol (Praha) 1978;23(5):366–371. doi: 10.1007/BF02876436. [DOI] [PubMed] [Google Scholar]
- Biely P., Krátký Z., Vrsanská M. Substrate-binding site of endo-1,4-beta-xylanase of the yeast Cryptococcus albidus. Eur J Biochem. 1981 Oct;119(3):559–564. doi: 10.1111/j.1432-1033.1981.tb05644.x. [DOI] [PubMed] [Google Scholar]
- Biely P., Krátký Z., Vrsanská M., Urmanicová D. Induction and inducers of endo-1,4-beta-xylanase in the yeast Cryptococcus albidus. Eur J Biochem. 1980;108(1):323–329. doi: 10.1111/j.1432-1033.1980.tb04726.x. [DOI] [PubMed] [Google Scholar]
- Biely P., Vrsanská M., Krátký Z. Mechanisms of substrate digestion by endo-1,4-beta-xylanase of Cryptococcus albidus. Lysozyme-type pattern of action. Eur J Biochem. 1981 Oct;119(3):565–571. doi: 10.1111/j.1432-1033.1981.tb05645.x. [DOI] [PubMed] [Google Scholar]
- Biely P., Vrsanská M., Krátký Z. Xylan-degrading enzymes of the yeast Cryptococcus albidus. Identification and cellular localization. Eur J Biochem. 1980;108(1):313–321. doi: 10.1111/j.1432-1033.1980.tb04725.x. [DOI] [PubMed] [Google Scholar]
- Dekker R. F., Richards G. N. Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem. 1976;32:277–352. doi: 10.1016/s0065-2318(08)60339-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Wickerham L. J., Kurtzman C. P. Synergistic color variants of Aureobasidium pullulans. Mycologia. 1975 Mar-Apr;67(2):342–361. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]