Abstract
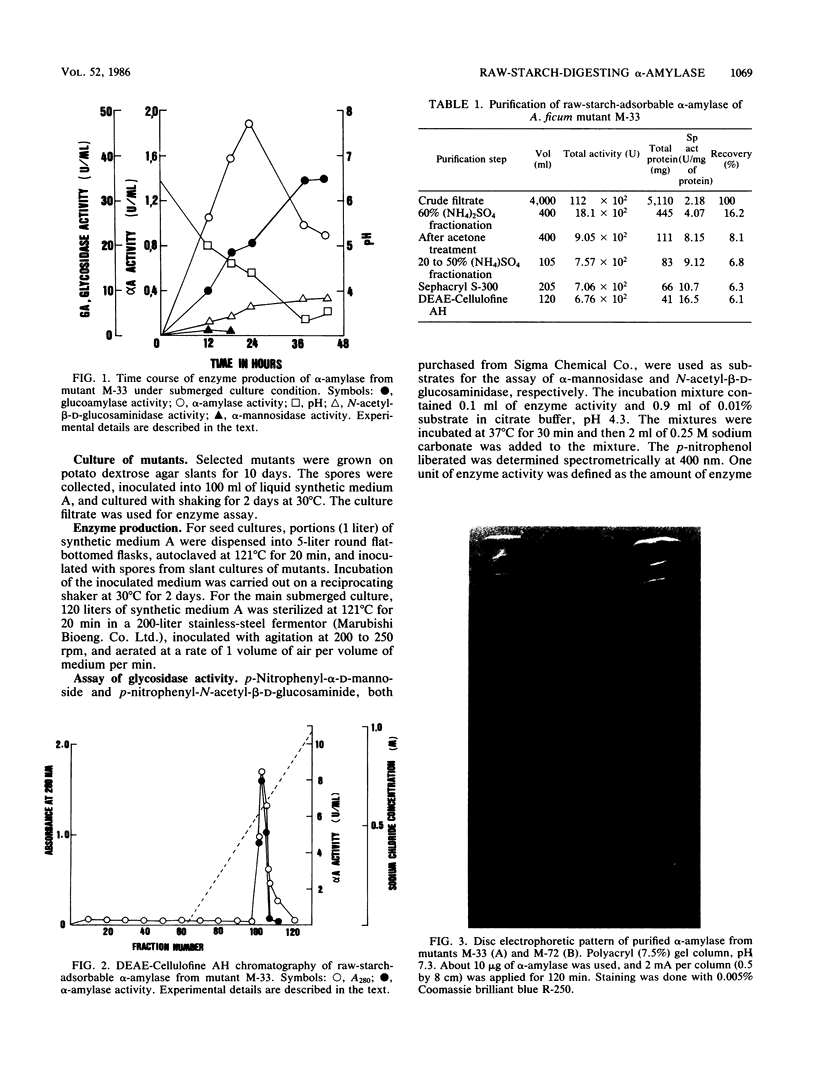
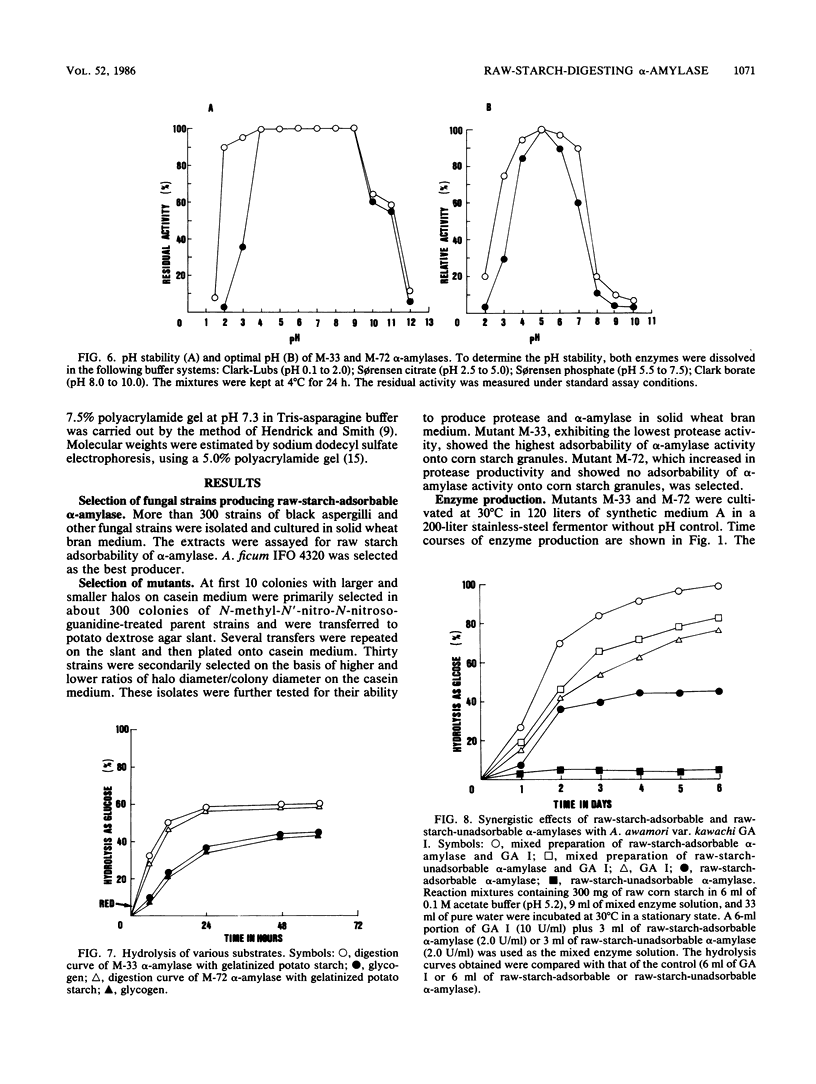
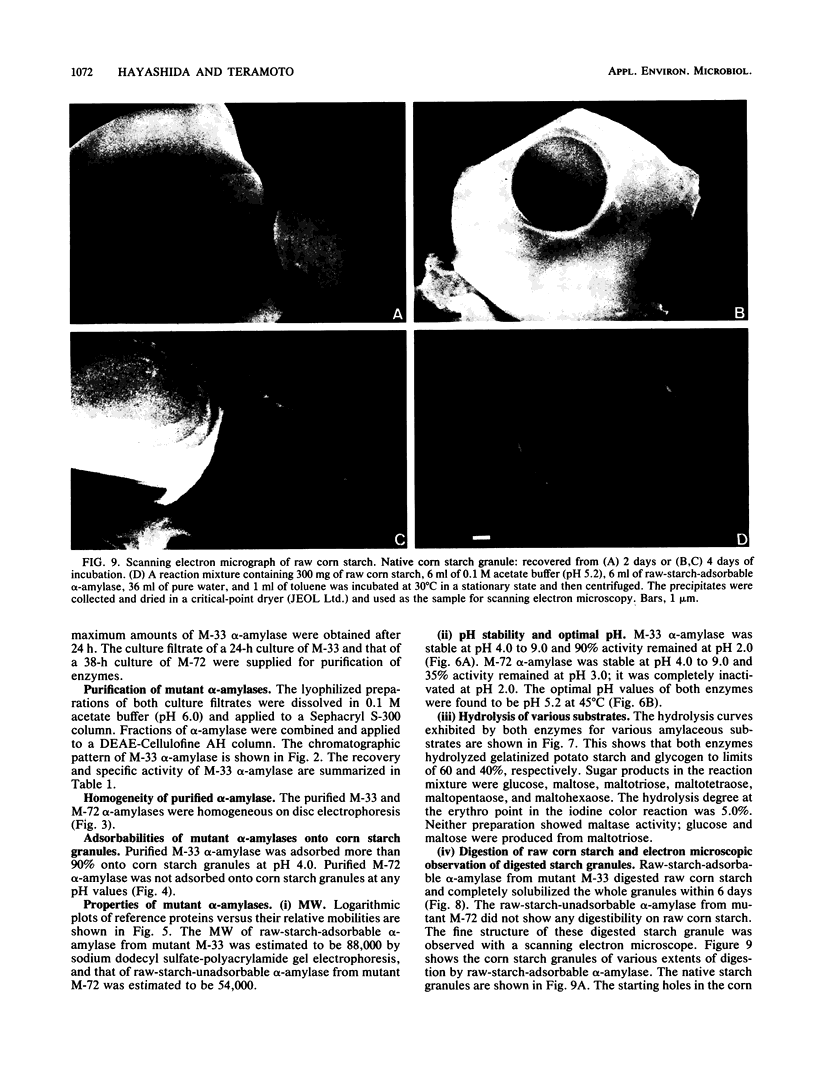
Mutational experiments were carried out to decrease the protease productivity of Aspergillus ficum IFO 4320 by using N-methyl-N′-nitro-N-nitrosoguanidine. A protease-negative mutant, M-33, exhibited higher α-amylaseactivity than the parent strain under submerged culture at 30°C for 24 h. About 70% of the total α-amylase activity in the M-33 culture filtrate was adsorbed onto starch granules. The electrophoretically homogeneous preparation of raw-starch-adsorbable α-amylase (molecular weight, 88,000), acid stable at pH 2, showed intensive raw-starch-digesting activity, dissolving corn starch granules completely. The preparation also exhibited a high synergistic effect with glucoamylase I. A mutant, M-72, with higher protease activity produced a raw cornstarch-unadsorbable α-amylase. The purified enzyme (molecular weight, 54,000), acid unstable, showed no digesting activity on raw corn starch and a lower synergistic effect with glucoamylase I in the hydrolysis of raw corn starch. The fungal α-amylase was therefore divided into two types, a novel type of raw-starch-digesting enzyme and a conventional type of raw-starch-nondigesting enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Flor P. Q., Hayashida S. Production and Characteristics of Raw Starch-Digesting Glucoamylase O from a Protease-Negative, Glycosidase-Negative Aspergillus awamori var. kawachi Mutant. Appl Environ Microbiol. 1983 Mar;45(3):905–912. doi: 10.1128/aem.45.3.905-912.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yamakawa Y., Okuyama T. Heterogeneity of crystalline Taka-amylase. J Biochem. 1973 Feb;73(2):447–454. [PubMed] [Google Scholar]





