Abstract
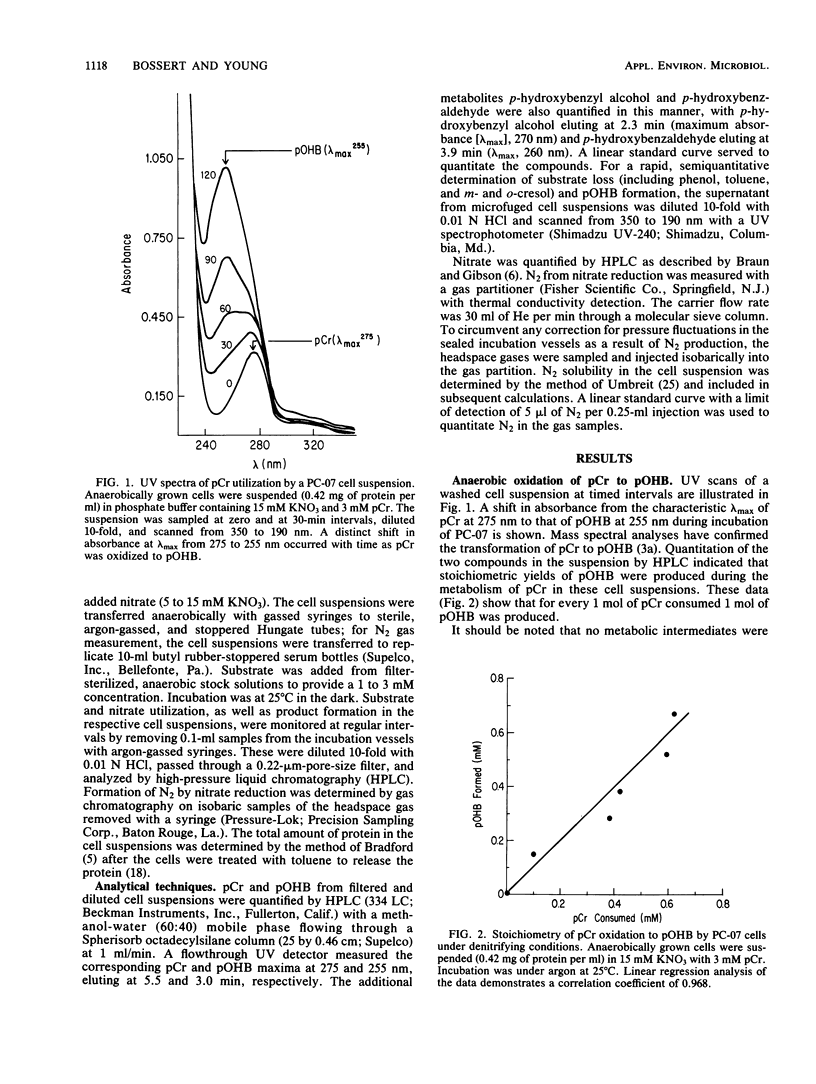
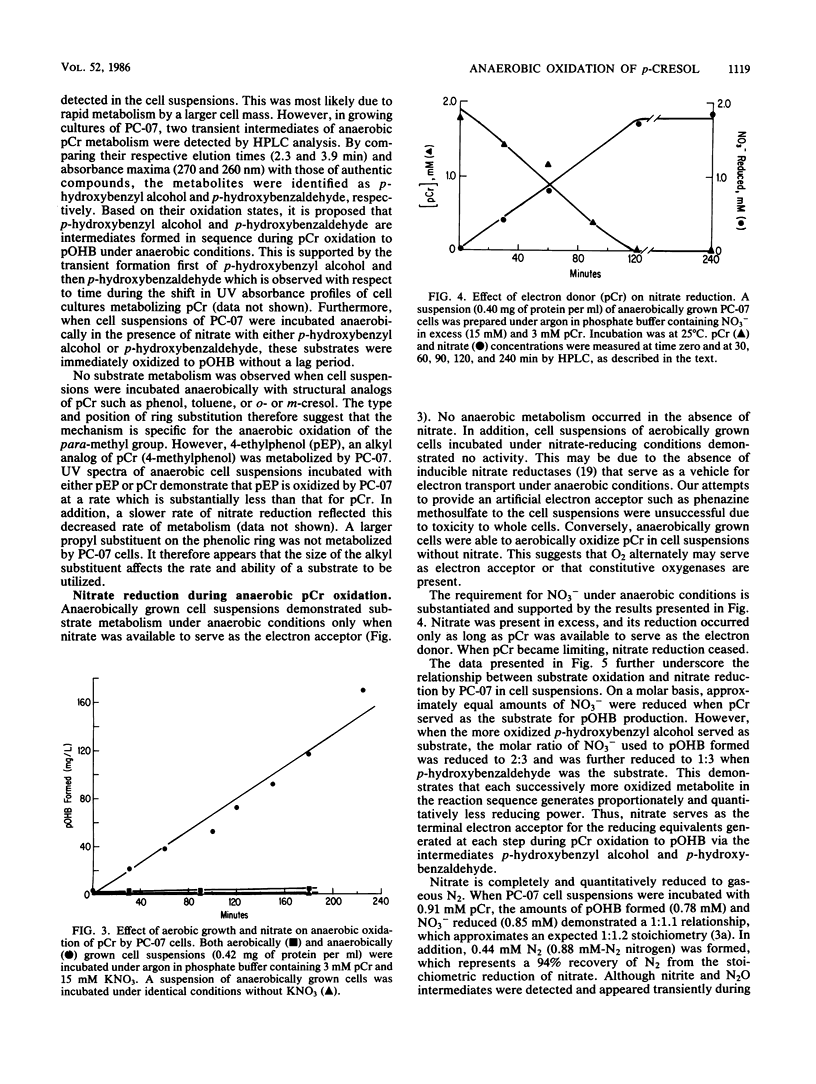
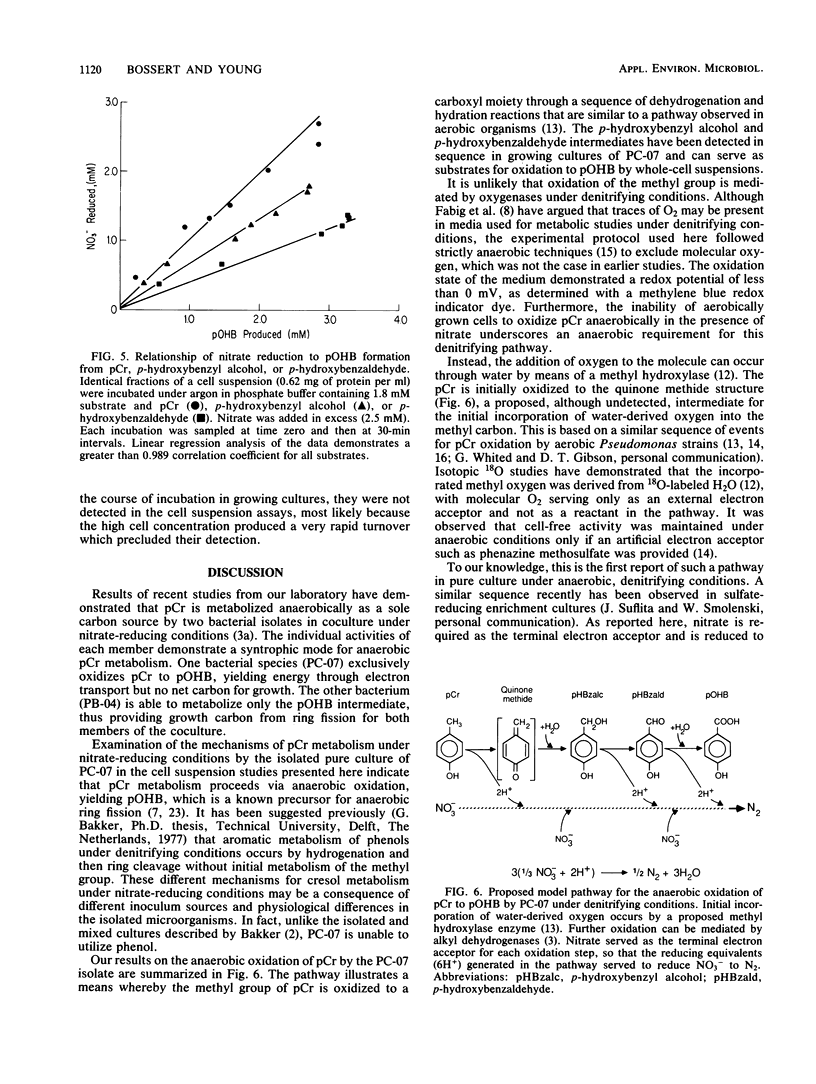
Metabolism of p-cresol (pCr) under nitrate-reducing conditions is mediated by the denitrifying bacterial isolate PC-07. The methyl substituent of the substrate is oxidized anaerobically by whole-cell suspensions of PC-07 through a series of dehydrogenation and hydration reactions to yield p-hydroxybenzoate (pOHB) in stoichiometric proportions. The partially oxidized intermediates in the pathway p-hydroxybenzyl alcohol and p-hydroxybenzaldehyde can also serve as substrates for pOHB formation. Nitrate is required as the external electron acceptor and is reduced to molecular N2. Reduction of the nitrate is stoichiometric, with pCr serving as the electron donor. In addition, the molar relationship between the electron acceptor (NO3-) reduced to the electron donor oxidized decreased to approximately 2:3 and then to 1:3 when p-hydroxybenzyl alcohol or p-hydroxybenzaldehyde, respectively, served as substrates. The decreased ratios were to be expected when the partially oxidized intermediates served as substrates, because they provided correspondingly less reducing power for pOHB formation. The anaerobic oxidation of pCr by PC-07 demonstrates a mechanism whereby aromatic compounds can be transformed in anoxic environments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftring R. P., Chalker B. E., Taylor B. F. Degradation of phthalic acids by denitrifying, mixed cultures of bacteria. Appl Environ Microbiol. 1981 May;41(5):1177–1183. doi: 10.1128/aem.41.5.1177-1183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R. Anaerobic biodegradation of chlorophenols in fresh and acclimated sludge. Appl Environ Microbiol. 1984 Feb;47(2):272–277. doi: 10.1128/aem.47.2.272-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun K., Gibson D. T. Anaerobic degradation of 2-aminobenzoate (anthranilic acid) by denitrifying bacteria. Appl Environ Microbiol. 1984 Jul;48(1):102–107. doi: 10.1128/aem.48.1.102-107.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Godsy E. M., Goerlitz D. F., Ehrlich G. G. Methanogenesis of phenolic compounds by a bacterial consortium from a contaminated aquifier in St. Louis Park, Minnesota. Bull Environ Contam Toxicol. 1983 Mar;30(3):261–268. doi: 10.1007/BF01610131. [DOI] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y. Anaerobic biodegradation of eleven aromatic compounds to methane. Appl Environ Microbiol. 1979 Jul;38(1):84–89. doi: 10.1128/aem.38.1.84-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J. Incorporation of [18O]water in the formation of p-hydroxybenzyl alcohol by the p-cresol methylhydroxylase from Pseudomonas putida. Biochem J. 1978 Oct 1;175(1):345–347. doi: 10.1042/bj1750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Taylor D. G. Pathways for the degradation of m-cresol and p-cresol by Pseudomonas putida. J Bacteriol. 1975 Apr;122(1):1–6. doi: 10.1128/jb.122.1.1-6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Taylor D. G. The purification and properties of p-cresol-(acceptor) oxidoreductase (hydroxylating), a flavocytochrome from Pseudomonas putida. Biochem J. 1977 Oct 1;167(1):155–162. doi: 10.1042/bj1670155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keat M. J., Hopper D. J. P-cresol and 3,5-xylenol methylhydroxylases in Pseudomonas putida N.C.I.B. 9896. Biochem J. 1978 Nov 1;175(2):649–658. doi: 10.1042/bj1750649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schennen U., Braun K., Knackmuss H. J. Anaerobic degradation of 2-fluorobenzoate by benzoate-degrading, denitrifying bacteria. J Bacteriol. 1985 Jan;161(1):321–325. doi: 10.1128/jb.161.1.321-325.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat R., Robinson J. P. The bacteriology of anaerobic degradation of aromatic compounds. J Appl Bacteriol. 1984 Dec;57(3):381–394. doi: 10.1111/j.1365-2672.1984.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Taylor B. F. Aerobic and Anaerobic Catabolism of Vanillic Acid and Some Other Methoxy-Aromatic Compounds by Pseudomonas sp. Strain PN-1. Appl Environ Microbiol. 1983 Dec;46(6):1286–1292. doi: 10.1128/aem.46.6.1286-1292.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Campbell W. L., Chinoy I. Anaerobic degradation of the benzene nucleus by a facultatively anaerobic microorganism. J Bacteriol. 1970 May;102(2):430–437. doi: 10.1128/jb.102.2.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Hearn W. L., Pincus S. Metabolism of monofluoro- and monochlorobenzoates by a dentrifying bacterium. Arch Microbiol. 1979 Sep;122(3):301–306. doi: 10.1007/BF00411295. [DOI] [PubMed] [Google Scholar]
- Williams R. J., Evans W. C. The metabolism of benzoate by Moraxella species through anaerobic nitrate respiration. Evidence for a reductive pathway. Biochem J. 1975 Apr;148(1):1–10. doi: 10.1042/bj1480001a. [DOI] [PMC free article] [PubMed] [Google Scholar]



