Abstract
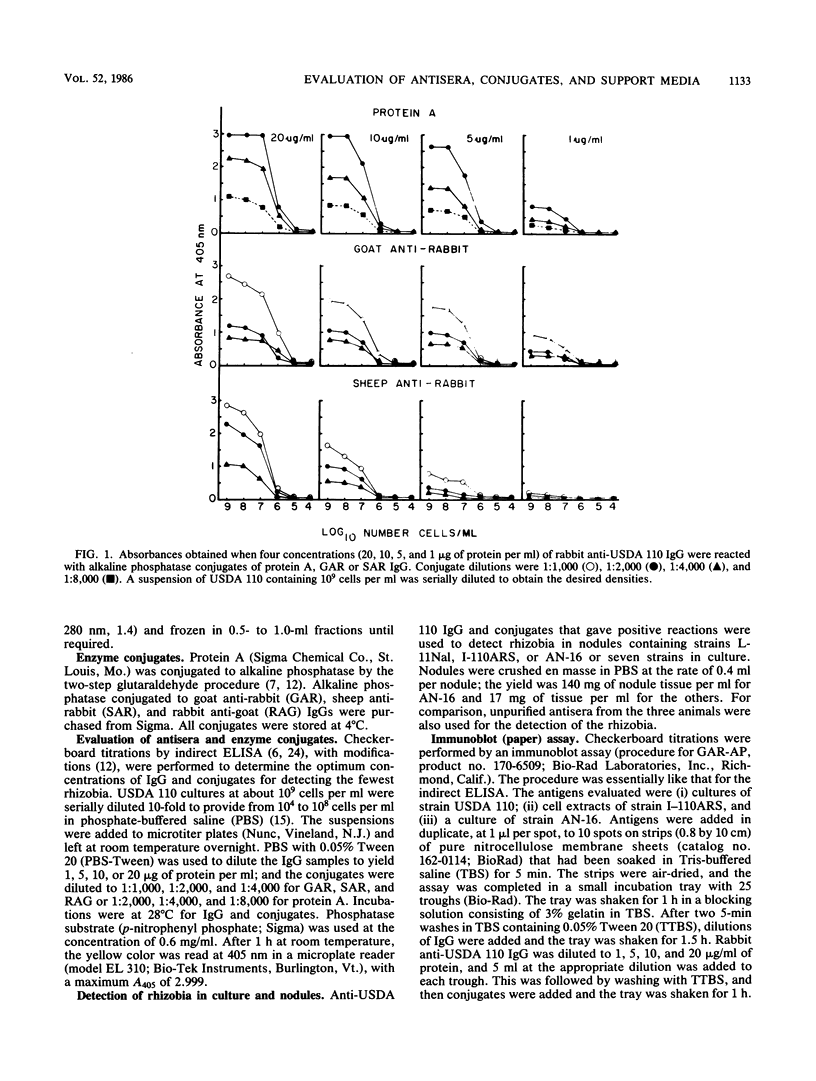
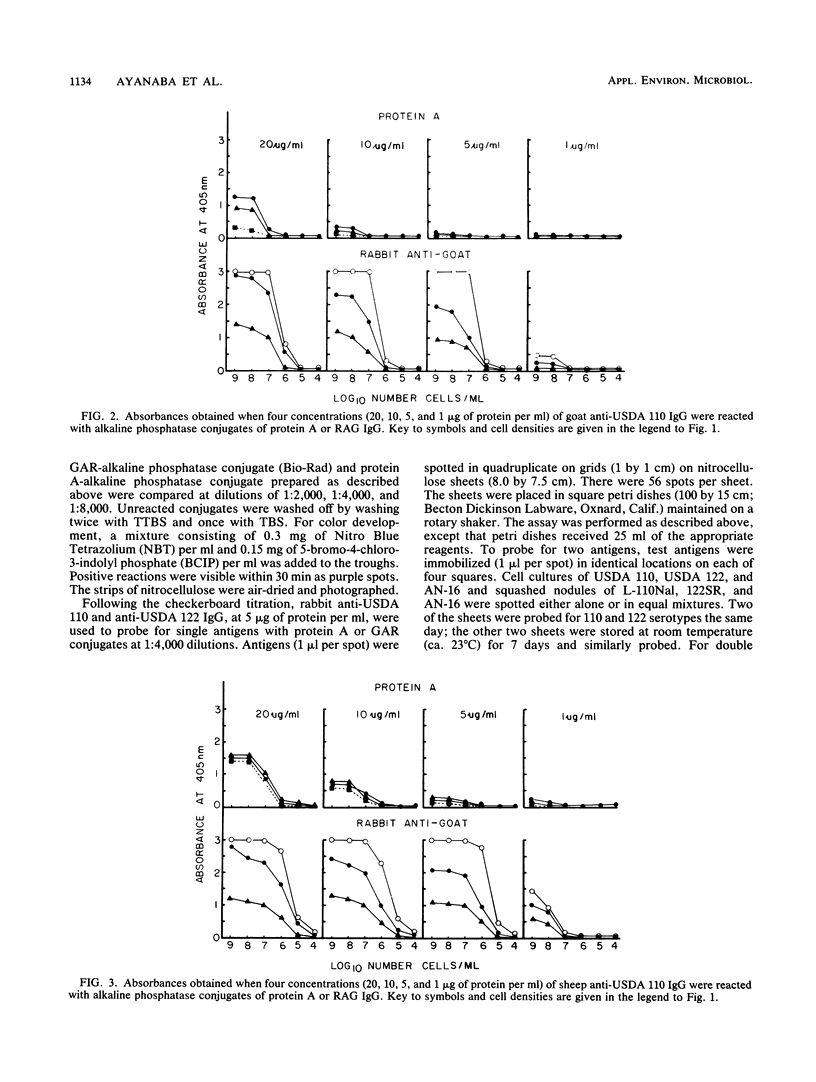
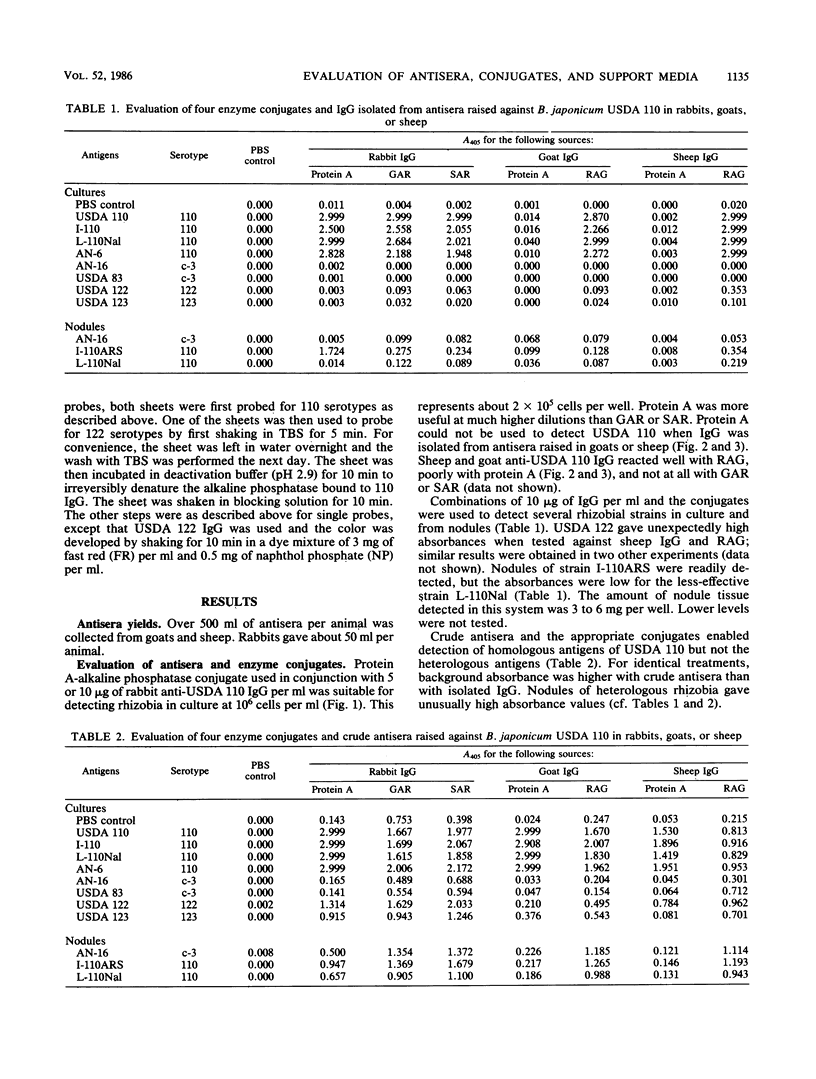
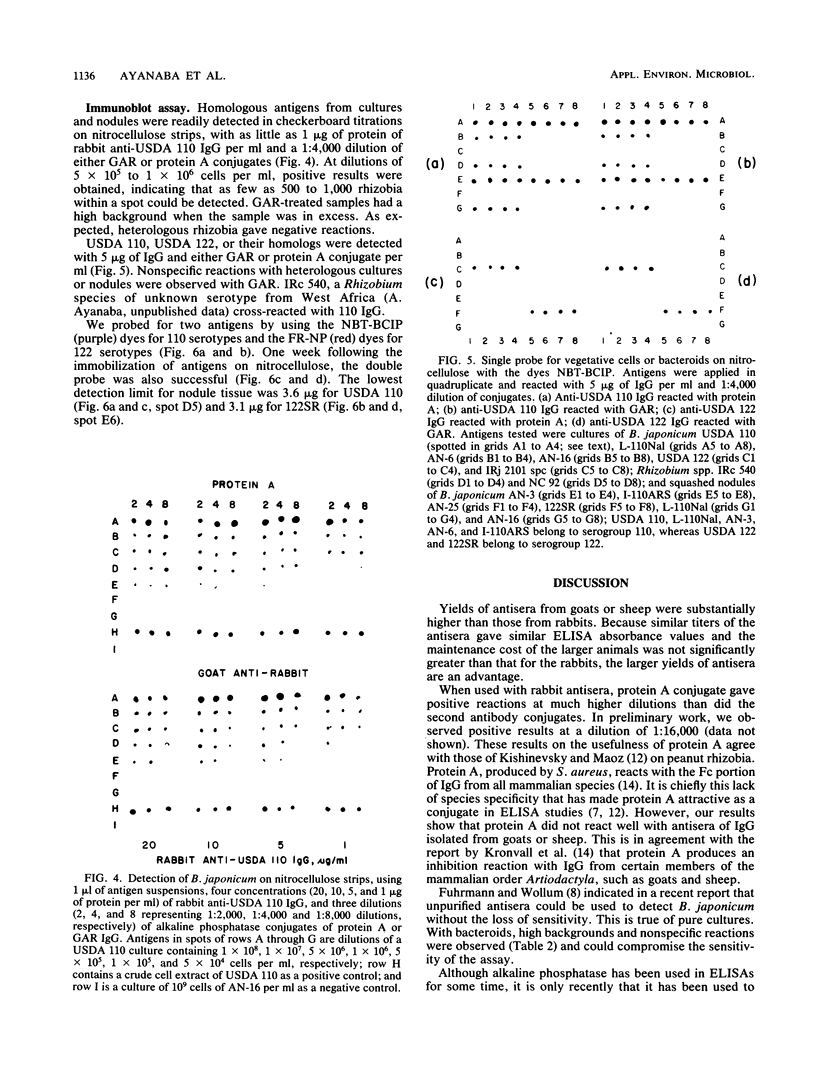
We evaluated three antisera and four enzyme conjugates for the detection of Bradyrhizobium japonicum by an indirect enzyme-linked immunosorbent assay in microtiter plates. Nitrocellulose membrane sheets were then evaluated as an alternative support medium by using some combinations. Partially purified immunoglobulin G (IgG) or unpurified antisera to strain USDA 110 raised in rabbits, goats, or sheep was reacted in microtiter plates with alkaline phosphatase conjugated to protein A, goat anti-rabbit (GAR), sheep anti-rabbit (SAR), or rabbit anti-goat (RAG) IgG. Cultures or nodules containing homologous rhizobia were detected with equal sensitivity when protein A, GAR, or SAR was reacted with 5 μg of protein IgG per ml or a 1:800 titer of antisera from rabbits, but not goats or sheep. RAG reacted with IgG or antisera from goats or sheep. The detection limit was 2 × 105 rhizobia per well. Rhizobia were spotted on nitrocellulose sheets as an alternative support medium, followed by soaking in 5 μg of protein per ml as IgG and 1:4,000 dilutions of protein A or GAR conjugate. Rhizobia in serogroup 110 were detected with the dye combination Nitro Blue Tetrazolium-5-bromo-4-chloro-3-indolyl phosphate (NBT-BCIP), and rhizobia in serogroup 122 were detected with fast red-naphthol phosphate (FR-NP). At the conclusion of the 5-h assay, purple (NBT-BCIP) or red (FR-NP) spots were visible in positive reactions. The sensitivity of detection was about 1,000 rhizobial cells or 3 μg of nodules tissue.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger J. A., May S. N., Berger L. R., Bohlool B. B. Colorimetric enzyme-linked immunosorbent assay for the identification of strains of Rhizobium in culture and in the nodules of lentils. Appl Environ Microbiol. 1979 Mar;37(3):642–646. doi: 10.1128/aem.37.3.642-646.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Clark M. F., Adams A. N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977 Mar;34(3):475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- Fuhrmann J., Wollum A. G. Simplified Enzyme-Linked Immunosorbent Assay for Routine Identification of Rhizobium japonicum Antigens. Appl Environ Microbiol. 1985 Apr;49(4):1010–1013. doi: 10.1128/aem.49.4.1010-1013.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Kishinevsky B., Bar-Joseph M. Rhizobium strain identification in Arachis hypogaea nodules by enzyme-linked immunosorbent assay (ELISA). Can J Microbiol. 1978 Dec;24(12):1537–1543. doi: 10.1139/m78-245. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Visualization of antigenic proteins on Western blots. Anal Biochem. 1984 Jan;136(1):180–184. doi: 10.1016/0003-2697(84)90321-x. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Seal U. S., Finstad J., Williams R. C., Jr Phylogenetic insight into evolution of mammalian Fc fragment of gamma G globulin using staphylococcal protein A. J Immunol. 1970 Jan;104(1):140–147. [PubMed] [Google Scholar]
- Mandrell R. E., Zollinger W. D. Use of a zwitterionic detergent for the restoration of the antibody-binding capacity of electroblotted meningococcal outer membrane proteins. J Immunol Methods. 1984 Feb 24;67(1):1–11. doi: 10.1016/0022-1759(84)90080-2. [DOI] [PubMed] [Google Scholar]
- O'Connor C. G., Ashman L. K. Application of the nitrocellulose transfer technique and alkaline phosphatase conjugated anti-immunoglobulin for determination of the specificity of monoclonal antibodies to protein mixtures. J Immunol Methods. 1982 Oct 29;54(2):267–271. doi: 10.1016/0022-1759(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]