Abstract
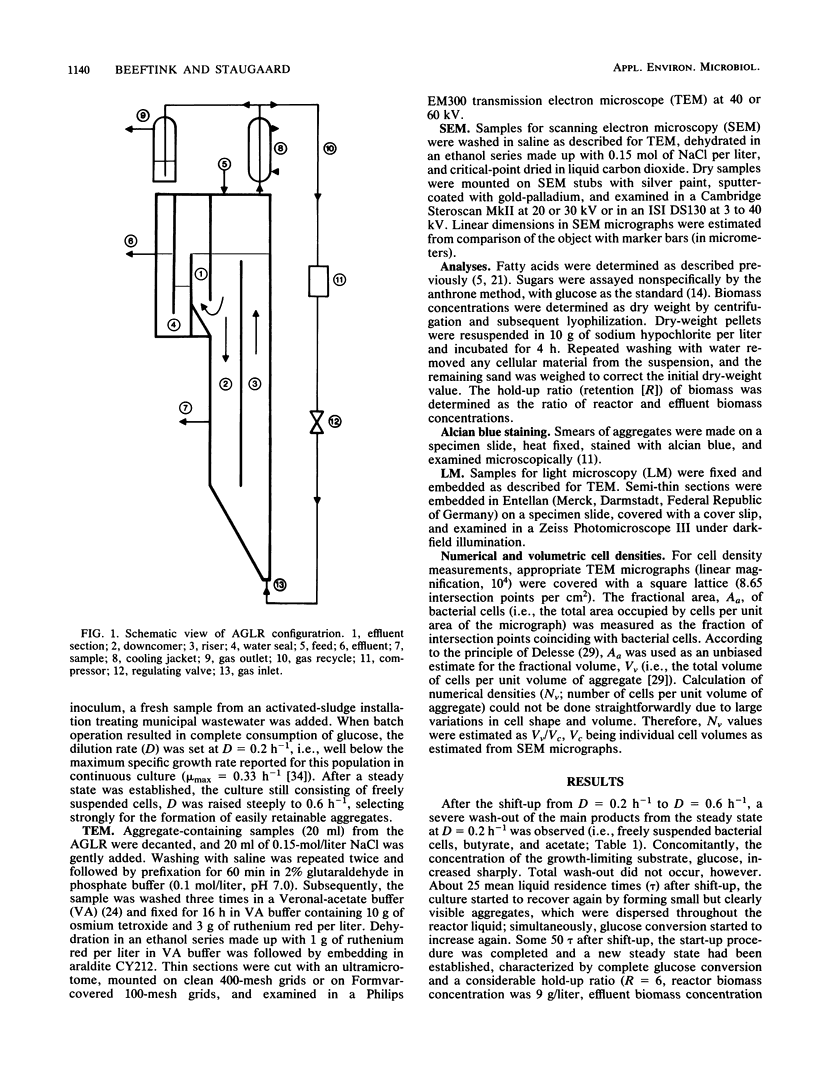
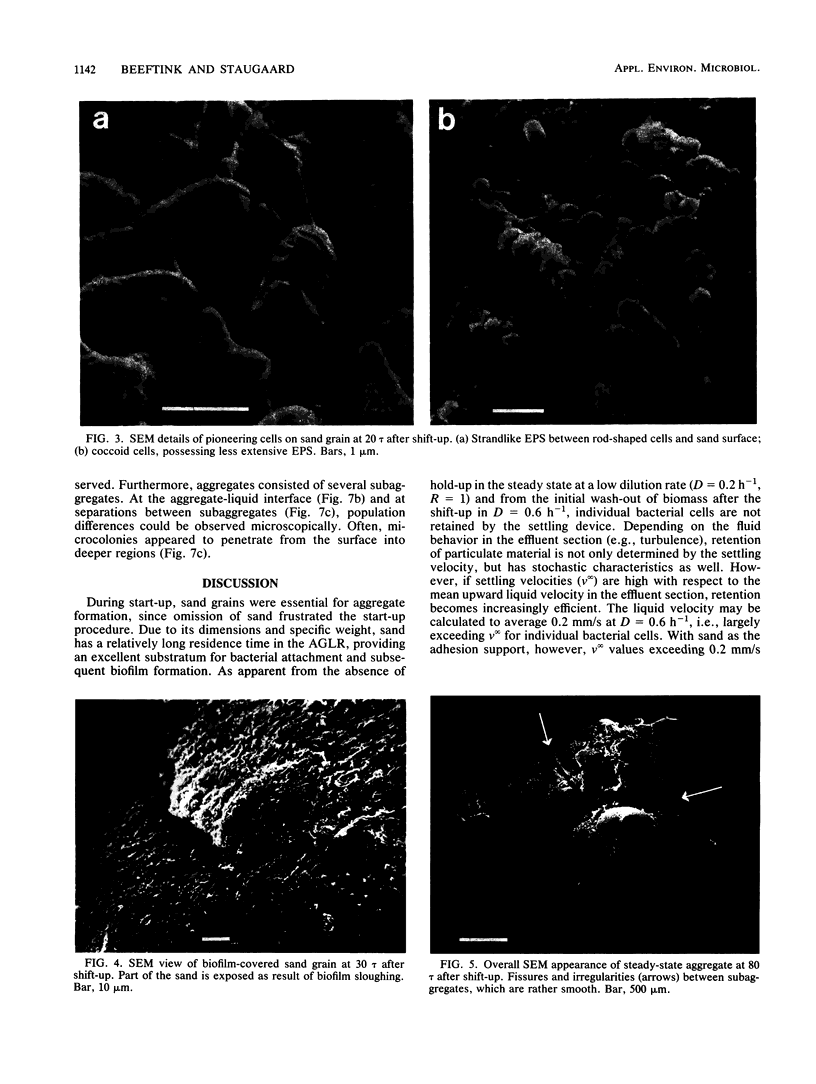
Anaerobic mixed-culture aggregates, which converted glucose to acetic, propionic, butyric, and valeric acids, were formed under controlled conditions of substrate feed (carbon limitation) and hydraulic regimen. The continuous-flow system used (anaerobic gas-lift reactor) was designed to retain bacterial aggregates in a well-mixed reactor. Carrier availability (i.e., liquid-suspended sand grains) proved necessary for bacterial aggregate formation from individual cells during reactor start-up. Electron microscopic examination revealed that incipient colonization of sand grains by bacteria from the bulk liquid occurred in surface irregularities, conceivably reflecting local quiescence. Subsequent confluent biofilm formation on sand grains proved to be unstable, however. Substrate depletion in the bulk liquid is assumed to weaken deeper parts of the biofilm due to cellular lysis, after which production of gas bubbles and liquid shearing forces cause sloughing. The resulting fragments, although sand free, were nevertheless large enough to be retained in the reactor and gradually grew larger through bacterial growth and by clumping together with other fragments. In the final steady state, high cell densities were maintained in the form of aggregates, while sand had virtually disappeared due to sampling losses and wash-out. Numerical cell densities within aggregates ranged from 1012/ml at the periphery to very low values in the center. The cells were enmeshed in a polymer matrix containing polysaccharides; nevertheless, carbon sufficiency was not a prerequisite to sustain high hold-up ratios.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costerton J. W., Geesey G. G., Cheng K. J. How bacteria stick. Sci Am. 1978 Jan;238(1):86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- Deinema M. H., Zevenhuizen L. P. Formation of cellulose fibrils by gram-negative bacteria and their role in bacterial flocculation. Arch Mikrobiol. 1971;78(1):42–51. doi: 10.1007/BF00409087. [DOI] [PubMed] [Google Scholar]
- Eighmy T. T., Maratea D., Bishop P. L. Electron microscopic examination of wastewater biofilm formation and structural components. Appl Environ Microbiol. 1983 Jun;45(6):1921–1931. doi: 10.1128/aem.45.6.1921-1931.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermyn M. A. Increasing the sensitivity of the anthrone method for carbohydrate. Anal Biochem. 1975 Sep;68(1):332–335. doi: 10.1016/0003-2697(75)90713-7. [DOI] [PubMed] [Google Scholar]
- Kierstan M., Bucke C. The immobilization of microbial cells, subcellular organelles, and enzymes in calcium alginate gels. Biotechnol Bioeng. 1977 Mar;19(3):387–397. doi: 10.1002/bit.260190309. [DOI] [PubMed] [Google Scholar]
- Kinner N. E., Balkwill D. L., Bishop P. L. Light and electron microscopic studies of microorganisms growing in rotating biological contactor biofilms. Appl Environ Microbiol. 1983 May;45(5):1659–1669. doi: 10.1128/aem.45.5.1659-1669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Robinson R. W., Akin D. E., Nordstedt R. A., Thomas M. V., Aldrich H. C. Light and electron microscopic examinations of methane-producing biofilms from anaerobic fixed-bed reactors. Appl Environ Microbiol. 1984 Jul;48(1):127–136. doi: 10.1128/aem.48.1.127-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago Y., Aida K. Exocellular mucopolysaccharide closely related to bacterial floc formation. Appl Environ Microbiol. 1977 Sep;34(3):308–314. doi: 10.1128/aem.34.3.308-314.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. G., Wimpenny J. W. Exopolysaccharide production by Pseudomonas NCIB11264 grown in continuous culture. J Gen Microbiol. 1978 Jan;104(1):47–57. doi: 10.1099/00221287-104-1-47. [DOI] [PubMed] [Google Scholar]
- Zevenhuizen L. P. Cellular glycogen, beta-1,2,-glucan, poly beta-hydroxybutyric acid and extracellular polysaccharides in fast-growing species of Rhizobium. Antonie Van Leeuwenhoek. 1981;47(6):481–497. doi: 10.1007/BF00443236. [DOI] [PubMed] [Google Scholar]