Abstract
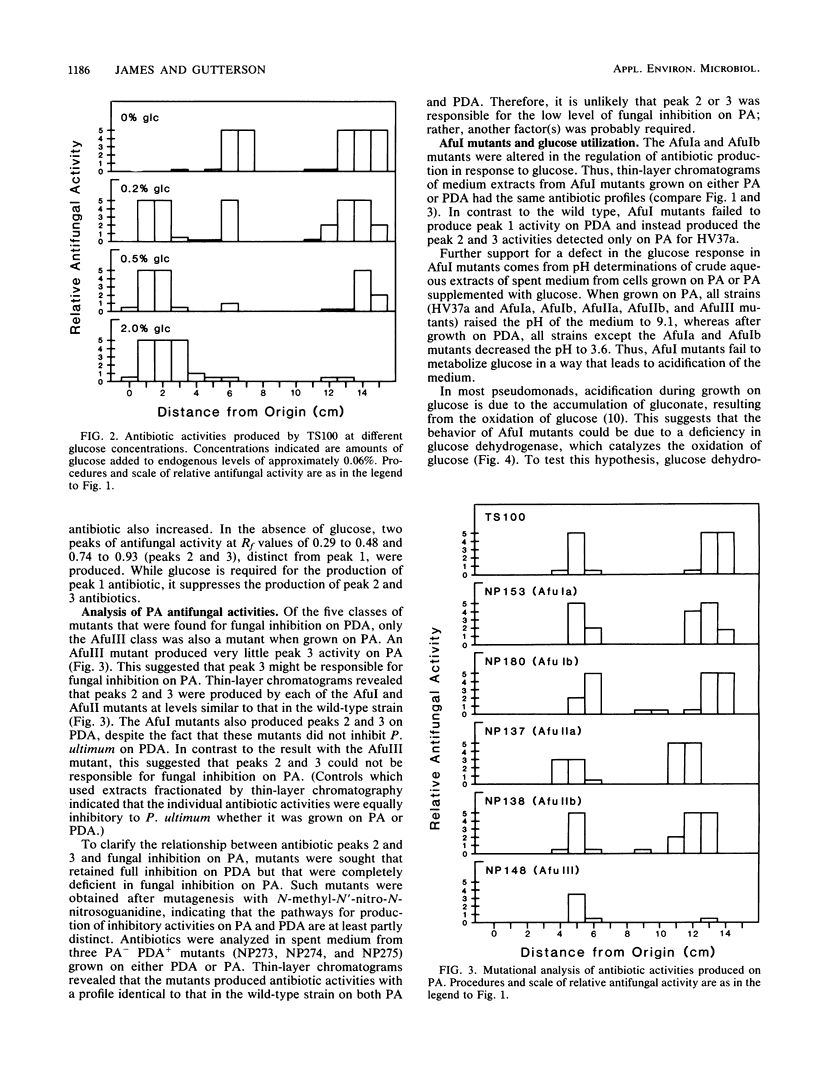
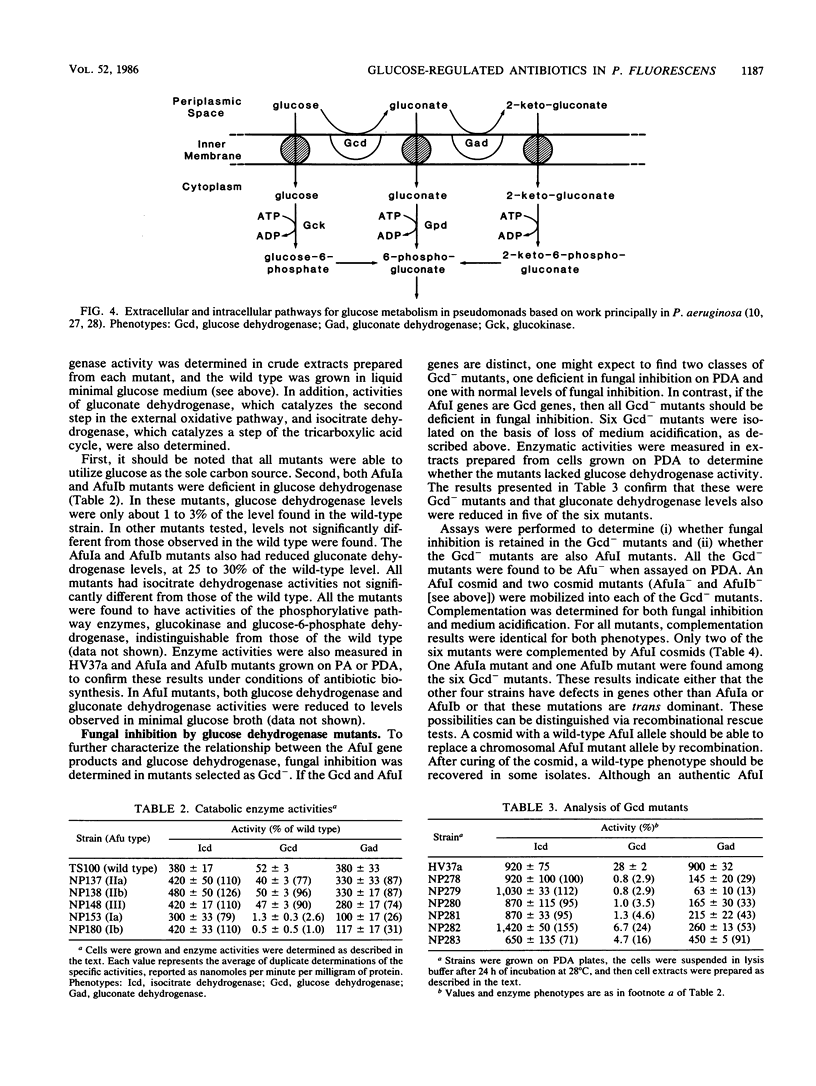
Pseudomonas fluorescens HV37a inhibited growth of the fungus Pythium ultimum on potato dextrose agar (PDA). An antibiotic activity produced under these conditions was fractionated and partially characterized. Extracts prepared from the PDA on which HV37a was grown revealed a single peak of antibiotic activity on thin-layer chromatograms. Similar extracts were prepared from mutants of HV37a. Their analysis indicated that the antibiotic observed in thin-layer chromatograms was responsible for fungal inhibition observed on PDA. The production of the PDA antibiotic required the presence of glucose, whereas two other antibiotic activities were produced only on potato agar without added glucose. Two mutants (denoted AfuIa and AfuIb) previously characterized as deficient in fungal inhibition on PDA showed altered regulation of the production of all three antibiotics in response to glucose. These mutants were also deficient in glucose dehydrogenase. Mutants isolated as deficient in glucose dehydrogenase were also deficient in fungal inhibition and were grouped into two classes on the basis of complementation analysis with an AfuI cosmid. Glucose regulation of antibiotic biosynthesis therefore involves at least two components and requires glucose dehydrogenase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuskey S. M., Phibbs P. V., Jr Chromosomal mapping of mutations affecting glycerol and glucose catabolism in Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Jun;162(3):872–880. doi: 10.1128/jb.162.3.872-880.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuskey S. M., Wolff J. A., Phibbs P. V., Jr, Olsen R. H. Cloning of genes specifying carbohydrate catabolism in Pseudomonas aeruginosa and Pseudomonas putida. J Bacteriol. 1985 Jun;162(3):865–871. doi: 10.1128/jb.162.3.865-871.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutterson N. I., Layton T. J., Ziegle J. S., Warren G. J. Molecular cloning of genetic determinants for inhibition of fungal growth by a fluorescent pseudomonad. J Bacteriol. 1986 Mar;165(3):696–703. doi: 10.1128/jb.165.3.696-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. H., Hopwood D. A. Molecular cloning and expression of the phenoxazinone synthase gene from Streptomyces antibioticus. J Biol Chem. 1984 Nov 25;259(22):14151–14157. [PubMed] [Google Scholar]
- Jones G. H. Regulation of phenoxazinone synthase expression in Streptomyces antibioticus. J Bacteriol. 1985 Sep;163(3):1215–1221. doi: 10.1128/jb.163.3.1215-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T. G., Phibbs P. V., Jr Alternative pathways of carbohydrate utilization in pseudomonads. Annu Rev Microbiol. 1984;38:359–388. doi: 10.1146/annurev.mi.38.100184.002043. [DOI] [PubMed] [Google Scholar]
- Lynch W. H., Franklin M. Effect of temperature on diauxic growth with glucose and organic acids in Pseudomonas fluorescens. Arch Microbiol. 1978 Aug 1;118(2):133–140. doi: 10.1007/BF00415721. [DOI] [PubMed] [Google Scholar]
- Lynch W. H., Franklin M. Effect of temperature on the uptake of glucose, gluconate, and 2-ketogluconate by Pseudomonas fluorescens. Can J Microbiol. 1978 Jan;24(1):56–62. doi: 10.1139/m78-010. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Ameyama M. D-Glucose dehydrogenase from Pseudomonas fluorescens, membrane-bound. Methods Enzymol. 1982;89(Pt 500):149–154. doi: 10.1016/s0076-6879(82)89026-5. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Shinagawa E., Ameyama M. D-Gluconate dehydrogenase from bacteria, 2-keto-D-gluconate-yielding, membrane-bound. Methods Enzymol. 1982;89(Pt 500):187–193. doi: 10.1016/s0076-6879(82)89033-2. [DOI] [PubMed] [Google Scholar]
- Ng F. M., Dawes E. A. Chemostat studies on the regulation of glucose metabolism in Pseudomonas aeruginosa by citrate. Biochem J. 1973 Feb;132(2):129–140. doi: 10.1042/bj1320129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAABO E., TERKILDSEN T. C. On the enzymatic determination of blood glucose. Scand J Clin Lab Invest. 1960;12(4):402–407. doi: 10.3109/00365516009065404. [DOI] [PubMed] [Google Scholar]
- Schroth M. N., Hancock J. G. Selected topics in biological control. Annu Rev Microbiol. 1981;35:453–476. doi: 10.1146/annurev.mi.35.100181.002321. [DOI] [PubMed] [Google Scholar]
- Whiting P. H., Midgley M., Dawes E. A. The regulation of transport of glucose, gluconate and 2-oxogluconate and of glucose catabolism in Pseudomonas aeruginosa. Biochem J. 1976 Mar 15;154(3):659–668. doi: 10.1042/bj1540659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P. H., Midgley M., Dawes E. A. The role of glucose limitation in the regulation of the transport of glucose, gluconate and 2-oxogluconate, and of glucose metabolism in Pseudomonas aeruginosa. J Gen Microbiol. 1976 Feb;92(2):304–310. doi: 10.1099/00221287-92-2-304. [DOI] [PubMed] [Google Scholar]
- van Schie B. J., Hellingwerf K. J., van Dijken J. P., Elferink M. G., van Dijl J. M., Kuenen J. G., Konings W. N. Energy transduction by electron transfer via a pyrrolo-quinoline quinone-dependent glucose dehydrogenase in Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter calcoaceticus (var. lwoffi). J Bacteriol. 1985 Aug;163(2):493–499. doi: 10.1128/jb.163.2.493-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



