Abstract
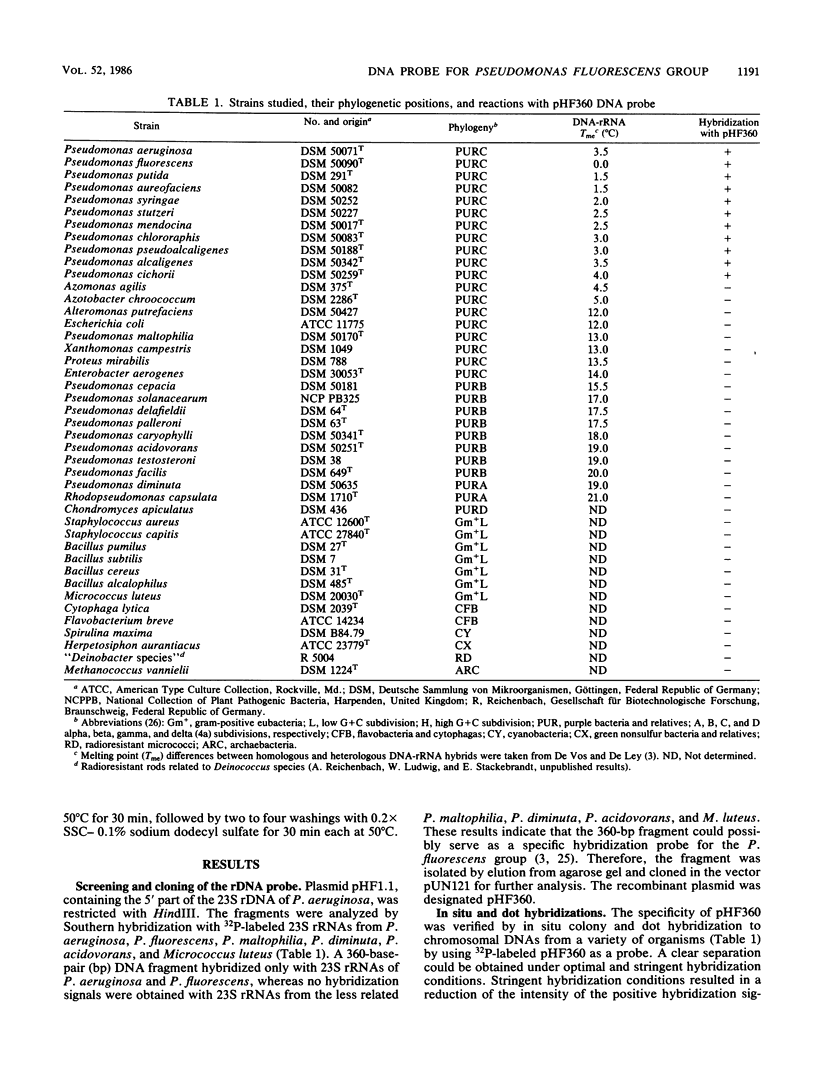
Plasmid pHF360 was constructed from cloned rRNA genes (rDNA) of Pseudomonas aeruginosa and used as hybridization probe for the Pseudomonas fluorescens group. The probe was tested by dot and in situ colony hybridizations to chromosomal DNAs from a wide variety of organisms. pHF360 DNA hybridized exclusively to chromosomal DNAs from bacteria representing the P. fluorescens group and separated them clearly from all other bacteria tested in the present study. Determination of the nucleotide sequence of the cloned DNA showed that it is a fragment from a 23S rRNA gene of P. aeruginosa. It was compared with the published 23S RNA sequence from Escherichia coli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas S. E., Doolittle W. F. Complete nucleotide sequence of the 23S rRNA gene of the Cyanobacterium, Anacystis nidulans. Nucleic Acids Res. 1984 Apr 11;12(7):3373–3386. doi: 10.1093/nar/12.7.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel U. B., Stanbridge E. J. Cloned mycoplasma ribosomal RNA genes for the detection of mycoplasma contamination in tissue cultures. Science. 1984 Dec 7;226(4679):1211–1213. doi: 10.1126/science.6505688. [DOI] [PubMed] [Google Scholar]
- Hennecke H., Günther I., Binder F. A novel cloning vector for the direct selection of recombinant DNA in E. coli. Gene. 1982 Sep;19(2):231–234. doi: 10.1016/0378-1119(82)90011-7. [DOI] [PubMed] [Google Scholar]
- Hennecke H., Günther I., Binder F. A novel cloning vector for the direct selection of recombinant DNA in E. coli. Gene. 1982 Sep;19(2):231–234. doi: 10.1016/0378-1119(82)90011-7. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kop J., Wheaton V., Gupta R., Woese C. R., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Bacillus stearothermophilus. DNA. 1984 Oct;3(5):347–357. doi: 10.1089/dna.1984.3.347. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Dees S. B. Cellular fatty acids and metabolic products of Pseudomonas species obtained from clinical specimens. J Clin Microbiol. 1976 Dec;4(6):492–502. doi: 10.1128/jcm.4.6.492-502.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B., Uhlén M., Josephson S., Gatenbeck S., Philipson L. An improved positive selection plasmid vector constructed by oligonucleotide mediated mutagenesis. Nucleic Acids Res. 1983 Nov 25;11(22):8019–8030. doi: 10.1093/nar/11.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett M. J., Pedersen M. M. Characterization of saccharolytic nonfermentative bacteria associated with man. Can J Microbiol. 1970 May;16(5):351–362. doi: 10.1139/m70-062. [DOI] [PubMed] [Google Scholar]
- Pickett M. J., Pedersen M. M. Salient features of nonsaccharolytic and weakly saccharolytic nonfermentative rods. Can J Microbiol. 1970 Jun;16(6):401–409. doi: 10.1139/m70-069. [DOI] [PubMed] [Google Scholar]
- Razin S., Gross M., Wormser M., Pollack Y., Glaser G. Detection of mycoplasmas infecting cell cultures by DNA hybridization. In Vitro. 1984 May;20(5):404–408. doi: 10.1007/BF02619586. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Lynn S. P., Gardner J. F. Use of randomly cloned DNA fragments for identification of Bacteroides thetaiotaomicron. J Bacteriol. 1983 Apr;154(1):287–293. doi: 10.1128/jb.154.1.287-293.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Stackebrandt E., Macke T. J., Fox G. E. A phylogenetic definition of the major eubacterial taxa. Syst Appl Microbiol. 1985;6:143–151. doi: 10.1016/s0723-2020(85)80047-3. [DOI] [PubMed] [Google Scholar]