Abstract
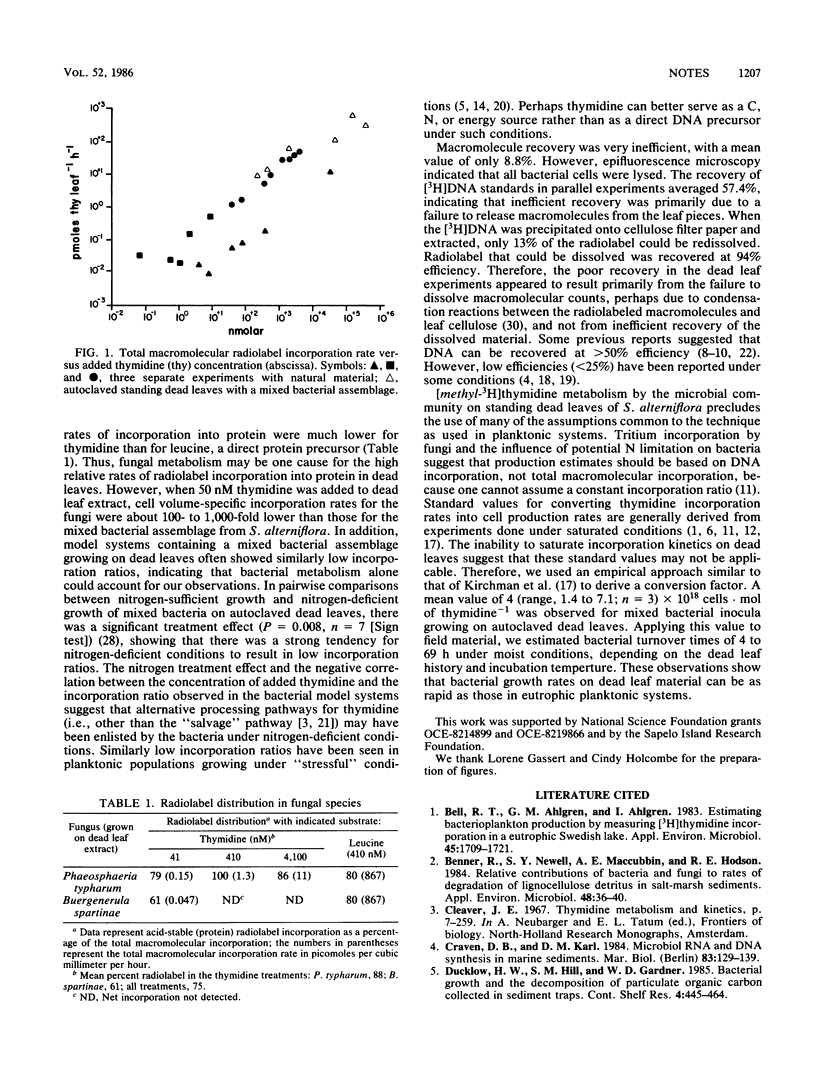
Thymidine incorporation by the microbial community on standing dead leaves of Spartina alterniflora did not obey many of the assumptions inherent in the use of the technique in planktonic systems. Incorporation rates of [methly-3H]thymidine were nonsaturable over a wide concentration range (101 to 105 nM). Owing to metabolism by both fungi and bacteria, a major fraction of the radiolabel (mean, 48%) appeared in protein. Extraction of the radiolabeled macromolecules was inefficient, averaging 8.8%. Based on an empirically derived conversion factor, 4 × 1018 cells · mol of thymidine−1, doubling times ranged from 4 to 69 h for the epiphytic bacterial assemblage.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner R., Newell S. Y., Maccubbin A. E., Hodson R. E. Relative contributions of bacteria and fungi to rates of degradation of lignocellulosic detritus in salt-marsh sediments. Appl Environ Microbiol. 1984 Jul;48(1):36–40. doi: 10.1128/aem.48.1.36-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eighmy T. T., Bishop P. L. Multiplicity of aspartate transport in thin wastewater biofilms. Appl Environ Microbiol. 1984 Dec;48(6):1151–1158. doi: 10.1128/aem.48.6.1151-1158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G. I., D'Ambrosio S. M., Jensen R. A. Versatile properties of a nonsaturable, homogeneous transport system in Bacilus subtilis: genetic, kinetic, and affinity labeling studies. Proc Natl Acad Sci U S A. 1975 Mar;72(3):814–818. doi: 10.1073/pnas.72.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Selected nucleic Acid precursors in studies of aquatic microbial ecology. Appl Environ Microbiol. 1982 Oct;44(4):891–902. doi: 10.1128/aem.44.4.891-902.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Primary and bacterial production in two dimictic indiana lakes. Appl Environ Microbiol. 1985 Mar;49(3):485–491. doi: 10.1128/aem.49.3.485-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley J. T., Konopka A. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annu Rev Microbiol. 1985;39:321–346. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]



