Abstract
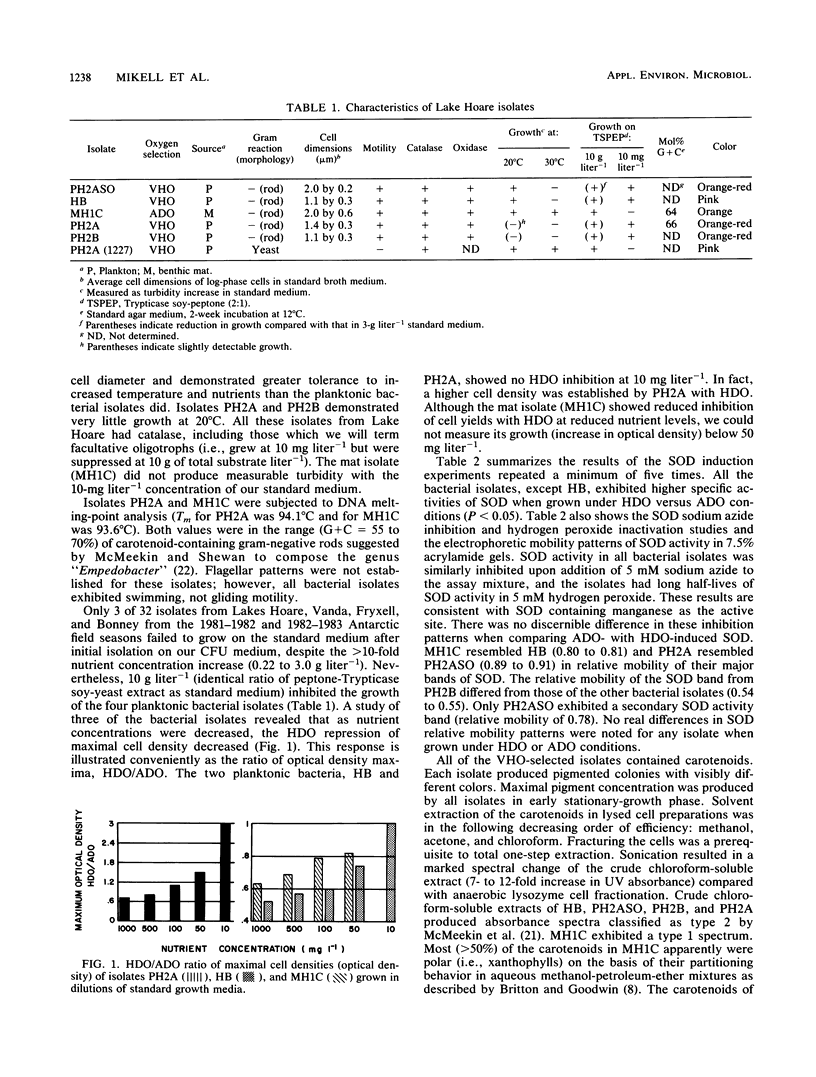
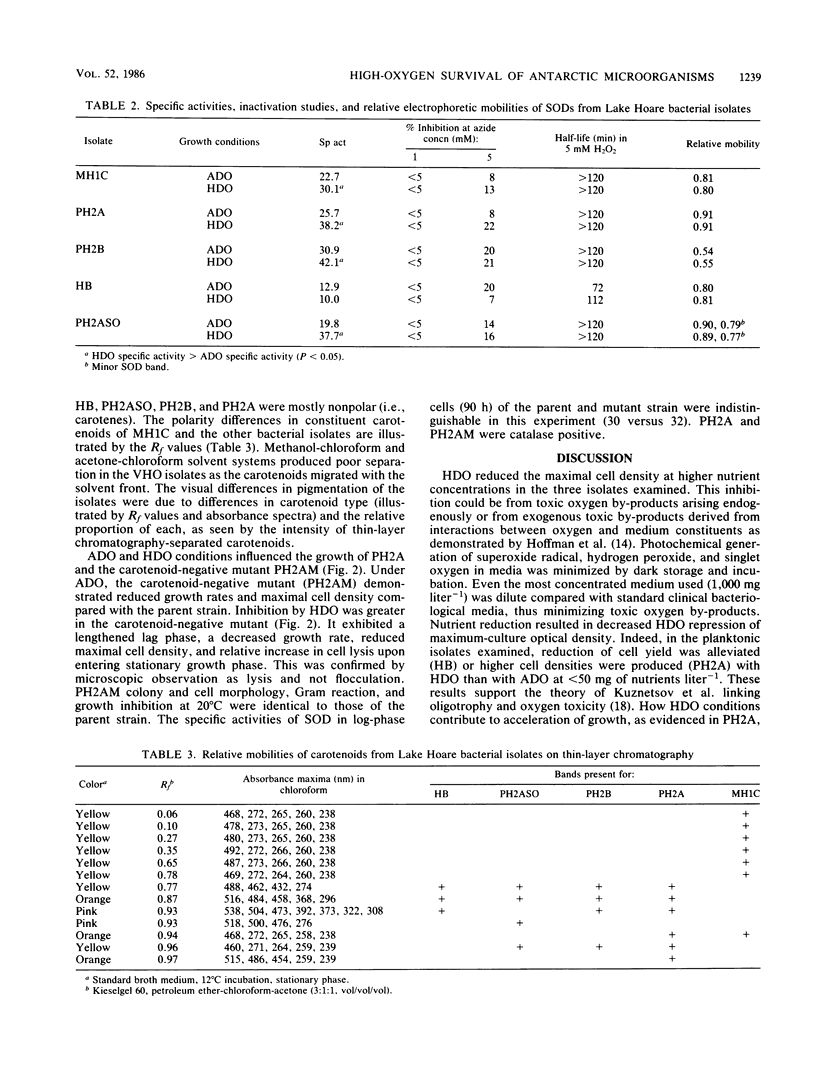
We sought to determine factors relating to the survival of heterotrophic microorganisms from the high-dissolved-oxygen (HDO) waters of Lake Hoare, Antarctica. This lake contains perpetual HDO about three times that of normal saturation (40 to 50 mg liter−1). Five isolates, one yeast and four bacteria, were selected from Lake Hoare waters by growth with the membrane filter technique with oxygen added to yield dissolved concentrations 14 times that in situ, 175 mg liter−1. One bacterial isolate was obtained from the microbial mat beneath the HDO waters. This organism was isolated at normal atmospheric oxygen saturation. The bacteria were gram-negative rods, motile, oxidase positive, catalase positive, and superoxide dismutase positive; they contained carotenoids. The planktonic isolates grew in media containing 10 mg of Trypticase soy (BBL Microbiology Systems)-peptone (2:1) liter−1 but not at 10 g liter−1. Under low-nutrient levels simulating Lake Hoare waters (10 mg liter−1), two of the planktonic isolates tested were not inhibited by HDO. Growth inhibition by HDO increased as nutrient concentration was increased. A carotenoid-negative mutant of one isolate demonstrated a decreased growth rate, maximal cell density, and increased cell lysis in the death phase under HDO compared with the parent strain. The specific activity of superoxide dismutase was increased by HDO in four of the five bacterial isolates. The superoxide dismutase was of the manganese type on the basis of inhibition and electrophoretic studies. The bacterial isolates from Lake Hoare possess several adaptations which may aid their survival in the HDO waters, as well as protection due to the oligotrophic nature of the lake.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Are growth rates of Escherichia coli in batch cultures limited by respiration? J Bacteriol. 1980 Oct;144(1):114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. M., Krinsky N. I. Protective action of carotenoid pigments against photodynamic damage to liposomes. Photochem Photobiol. 1973 Nov;18(5):403–408. doi: 10.1111/j.1751-1097.1973.tb06440.x. [DOI] [PubMed] [Google Scholar]
- Anderson S. M., Krinsky N. I., Stone M. J., Clagett D. C. Effect of singlet oxygen quenchers on oxidative damage to liposomes initiated by photosensitization or by radiofrequency discharge. Photochem Photobiol. 1974 Jul;20(1):65–69. doi: 10.1111/j.1751-1097.1974.tb06549.x. [DOI] [PubMed] [Google Scholar]
- Anwar M., Prebble J. The photoinactivation of the respiratory chain in Sarcina lutea (Micrococcus luteus) and protection by endogenous carotenoid. Photochem Photobiol. 1977 Nov;26(5):475–481. doi: 10.1111/j.1751-1097.1977.tb07517.x. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Burton G. W., Ingold K. U. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984 May 11;224(4649):569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. S., George H. A., Krieg N. R., Smibert R. M. Studies of the microaerophilic nature of Campylobacter fetus subsp. jejuni. II. Role of exogenous superoxide anions and hydrogen peroxide. Can J Microbiol. 1979 Jan;25(1):8–16. doi: 10.1139/m79-002. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Liu J. K., Moore E. R. Comparative Cytochrome Oxidase and Superoxide Dismutase Analyses on Strains of Azotobacter vinelandii and Other Related Free-Living Nitrogen-Fixing Bacteria. Appl Environ Microbiol. 1984 May;47(5):1185–1187. doi: 10.1128/aem.47.5.1185-1187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. I., Dubinina G. A., Lapteva N. A. Biology of oligotrophic bacteria. Annu Rev Microbiol. 1979;33:377–387. doi: 10.1146/annurev.mi.33.100179.002113. [DOI] [PubMed] [Google Scholar]
- Mandel M., Igambi L., Bergendahl J., Dodson M. L., Jr, Scheltgen E. Correlation of melting temperature and cesium chloride buoyant density of bacterial deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):333–338. doi: 10.1128/jb.101.2.333-338.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McMeekin T. A., Patterson J. T., Murray J. G. An initial approach to the taxonomy of some gram negative yellow pigmented rods. J Appl Bacteriol. 1971 Dec;34(4):699–716. doi: 10.1111/j.1365-2672.1971.tb01007.x. [DOI] [PubMed] [Google Scholar]
- McMeekin T. A., Shewan J. M. A review. Taxonomic strategies for Flavobacterium and related genera. J Appl Bacteriol. 1978 Dec;45(3):321–332. doi: 10.1111/j.1365-2672.1978.tb04232.x. [DOI] [PubMed] [Google Scholar]
- Mikell A. T., Parker B. C., Simmons G. M. Response of an antarctic lake heterotrophic community to high dissolved oxygen. Appl Environ Microbiol. 1984 May;47(5):1062–1066. doi: 10.1128/aem.47.5.1062-1066.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikell A. T., Parker B. C., Simmons G. M. Sensitivity of an oligotrophic lake planktonic bacterial community to oxygen stress. Appl Environ Microbiol. 1983 Sep;46(3):545–548. doi: 10.1128/aem.46.3.545-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra H. P., Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972 Jan 10;247(1):188–192. [PubMed] [Google Scholar]
- Singer C. E., Ames B. N. Sunlight ultraviolet and bacterial DNA base ratios. Science. 1970 Nov 20;170(3960):822–825. doi: 10.1126/science.170.3960.822. [DOI] [PubMed] [Google Scholar]
- Taylor R. F. Bacterial triterpenoids. Microbiol Rev. 1984 Sep;48(3):181–198. doi: 10.1128/mr.48.3.181-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirkell D., Hunter M. I. Carotenoid-glycoprotein of sarcina flava membrane. J Gen Microbiol. 1969 Nov;58(3):289–292. doi: 10.1099/00221287-58-3-289. [DOI] [PubMed] [Google Scholar]
- Von Stein R. S., Barber L. E., Hassan H. M. Biosynthesis of oxygen-detoxifying enzymes in Bdellovibrio stolpii. J Bacteriol. 1982 Nov;152(2):792–796. doi: 10.1128/jb.152.2.792-796.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


