Abstract
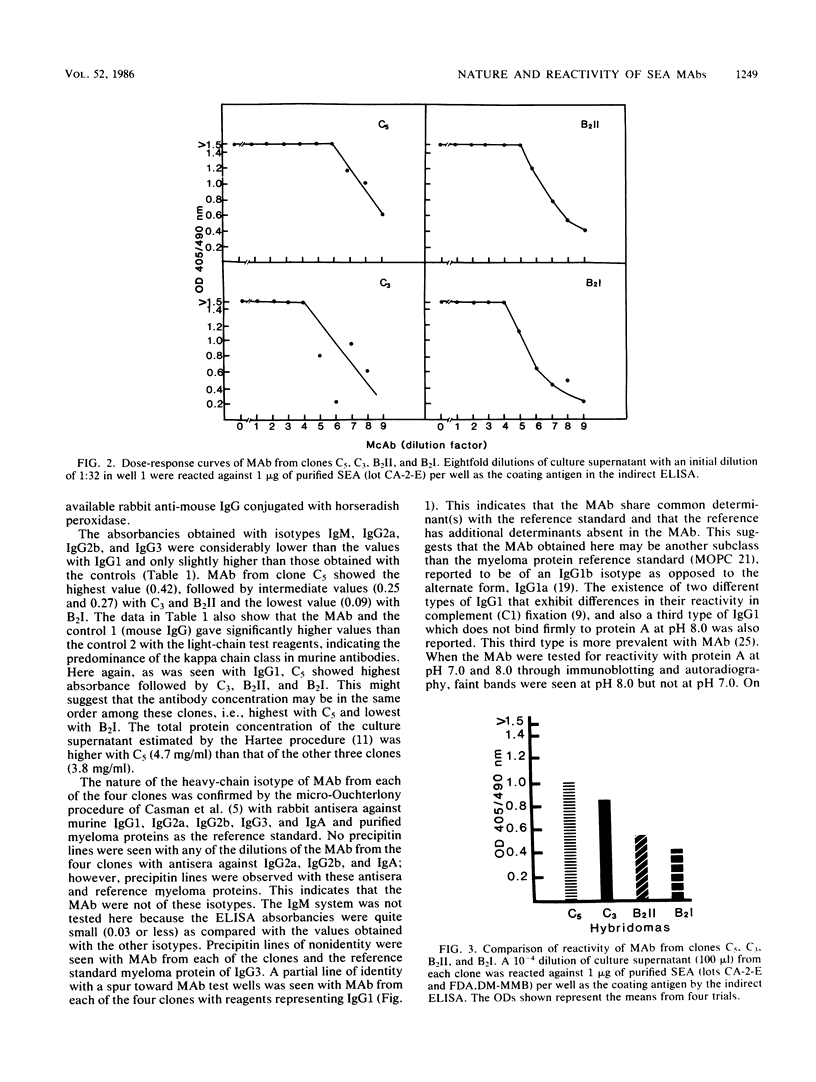
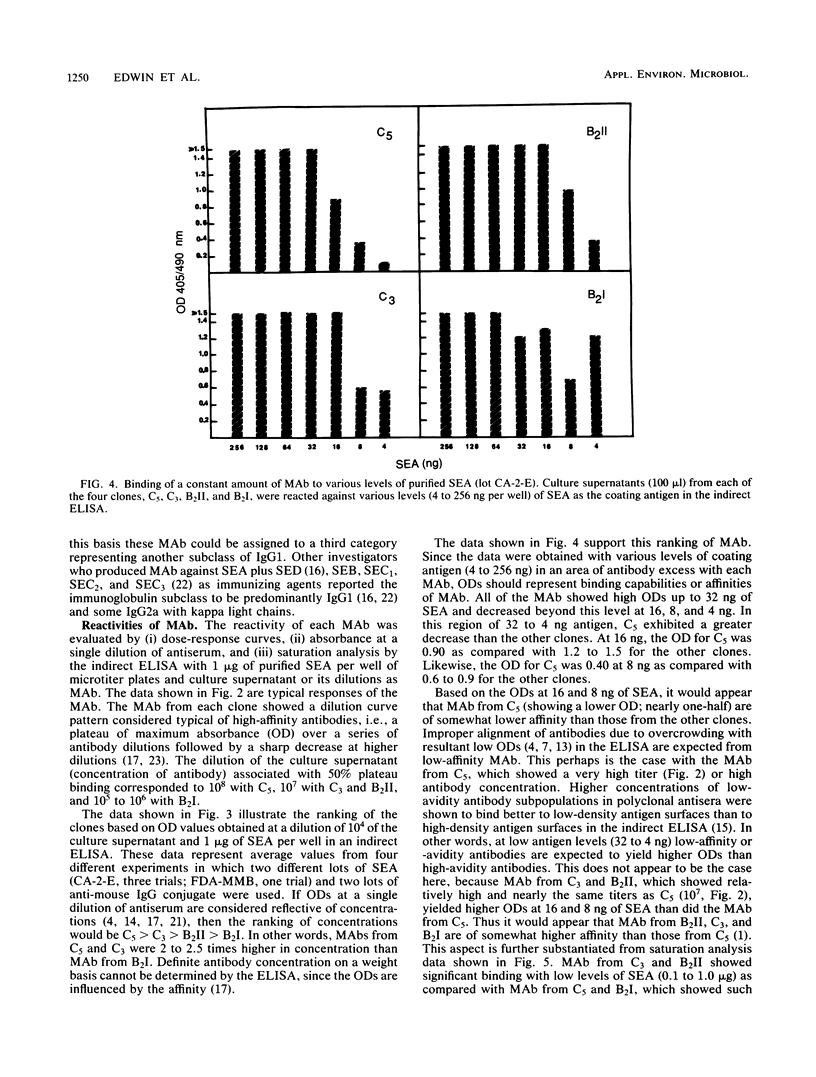
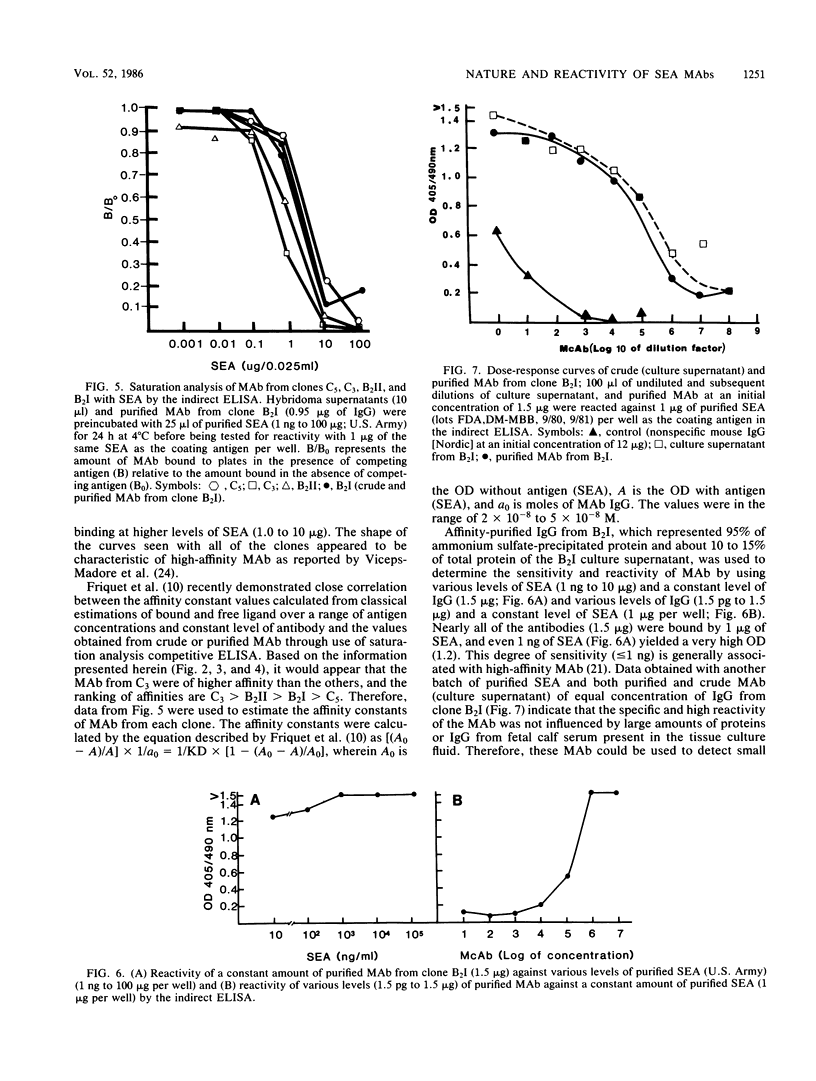
Monoclonal antibodies from four clones (C5, C3, B2II, and B2I) directed against staphylococcal enterotoxin A were tested by the indirect enzyme-linked immunosorbent assay and double-gel immunodiffusion (micro-Ouchterlony) assay for the nature of heavy and light chain types. The reactivities of monoclonal antibodies were also tested by indirect enzyme-linked immunosorbent assay with various levels of purified staphylococcal enterotoxin A and various levels (dilutions) of monoclonal antibodies and saturation analysis-competitive indirect enzyme-linked immunosorbent assay. The heavy-chain isotype of monoclonal antibodies was found to be an unspecified subclass of immunoglobulin G1, and the light chain was the kappa type. Monoclonal antibodies from all of the clones exhibited high reactivity and nearly the same affinity to staphylococcal enterotoxin A in saturation analysis-competitive enzyme-linked immunosorbent assay. Purified immunoglobulin G from B2I yielded very high absorbance (1.2) at 405 nm with 1 ng of staphylococcal enterotoxin A as the coating antigen in the enzyme-linked immunosorbent assay. Monoclonal antibodies from B2I also neutralized the biological activity of staphylococcal enterotoxin A when tested by the kitten bioassay.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlstedt S., Holmgren J., Hanson L. A. Protective capacity of antibodies against E. coli O antigen with special reference to the avidity. Int Arch Allergy Appl Immunol. 1974;46(3):470–480. doi: 10.1159/000231150. [DOI] [PubMed] [Google Scholar]
- Bird P., Lowe J., Stokes R. P., Bird A. G., Ling N. R., Jefferis R. The separation of human serum IgG into subclass fractions by immunoaffinity chromatography and assessment of specific antibody activity. J Immunol Methods. 1984 Jun 8;71(1):97–105. doi: 10.1016/0022-1759(84)90209-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruins S. C., Ingwer I., Zeckel M. L., White A. C. Parameters affecting the enzyme-linked immunosorbent assay of immunoglobulin G antibody to a rough mutant of Salmonella minnesota. Infect Immun. 1978 Sep;21(3):721–728. doi: 10.1128/iai.21.3.721-728.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casman E. P., Bennett R. W., Dorsey A. E., Stone J. E. The micro-slide gel double diffusion test for the detection and assay of staphylococcal enterotoxins. Health Lab Sci. 1969 Oct;6(4):185–198. [PubMed] [Google Scholar]
- Edwin C., Tatini S. R., Strobel R. S., Maheswaran S. K. Production of monoclonal antibodies to staphylococcal enterotoxin A. Appl Environ Microbiol. 1984 Dec;48(6):1171–1175. doi: 10.1128/aem.48.6.1171-1175.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Russell-Jones G. J., Jenkin C. R. Isotypes of mouse IgG--I. Evidence for 'non-complement-fixing' IgG1 antibodies and characterization of their capacity to interfere with IgG2 sensitization of target red blood cells for lysis by complement. Mol Immunol. 1980 Jun;17(6):699–710. doi: 10.1016/0161-5890(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods. 1985 Mar 18;77(2):305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Langone J. J. Applications of immobilized protein A in immunochemical techniques. J Immunol Methods. 1982 Dec 30;55(3):277–296. doi: 10.1016/0022-1759(82)90088-6. [DOI] [PubMed] [Google Scholar]
- Lehtonen O. P., Eerola E. The effect of different antibody affinities on ELISA absorbance and titer. J Immunol Methods. 1982 Oct 29;54(2):233–240. doi: 10.1016/0022-1759(82)90064-3. [DOI] [PubMed] [Google Scholar]
- Lehtonen O. P. Immunoreactivity of solid phase hapten binding plasmacytoma protein (ABPC 24). Mol Immunol. 1981 Apr;18(4):323–329. doi: 10.1016/0161-5890(81)90056-0. [DOI] [PubMed] [Google Scholar]
- Lehtonen O. P., Viljanen M. K. Antigen density in ELISA; effect on avidity dependency. J Immunol Methods. 1980;36(1):63–70. doi: 10.1016/0022-1759(80)90094-0. [DOI] [PubMed] [Google Scholar]
- Meyer R. F., Miller L., Bennett R. W., MacMillan J. D. Development of a monoclonal antibody capable of interacting with five serotypes of Staphylococcus aureus enterotoxin. Appl Environ Microbiol. 1984 Feb;47(2):283–287. doi: 10.1128/aem.47.2.283-287.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péterfy F., Kuusela P., Mäkelä O. Affinity requirements for antibody assays mapped by monoclonal antibodies. J Immunol. 1983 Apr;130(4):1809–1813. [PubMed] [Google Scholar]
- Russell S. H., Carter R., Firkin F. C. Determination of immunoglobulin class by pattern of absorption to protein A-sepharose coupled with immunoglobulin class specific antibodies. J Immunol Methods. 1984 Feb 10;66(2):323–326. doi: 10.1016/0022-1759(84)90344-2. [DOI] [PubMed] [Google Scholar]
- Stanislawski M., Mitard M. Recognition of two subclasses of mouse IgGl (gamma F). Immunochemistry. 1976 Dec;13(12):979–984. doi: 10.1016/0019-2791(76)90268-8. [DOI] [PubMed] [Google Scholar]
- Steensgaard J., Steward M. W., Frich J. R. The significance of antibody affinity heterogeneity in antigen-antibody reactions demonstrated by computer simulation. Mol Immunol. 1980 Jun;17(6):689–698. doi: 10.1016/0161-5890(80)90138-8. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Thompson N. E., Ketterhagen M. J., Bergdoll M. S. Monoclonal antibodies to staphylococcal enterotoxins B and C: cross-reactivity and localization of epitopes on tryptic fragments. Infect Immun. 1984 Jul;45(1):281–285. doi: 10.1128/iai.45.1.281-285.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen V., Brock D. J., Van Heyningen S. A simple method for ranking the affinities of monoclonal antibodies. J Immunol Methods. 1983 Aug 26;62(2):147–153. doi: 10.1016/0022-1759(83)90000-5. [DOI] [PubMed] [Google Scholar]
- Viceps-Madore D., Cidlowski J. A., Kittler J. M., Thanassi J. W. Preparation, characterization, and use of monoclonal antibodies to vitamin B6. J Biol Chem. 1983 Feb 25;258(4):2689–2696. [PubMed] [Google Scholar]
- Villemez C. L., Russell M. A., Carlo P. L. Mouse IgG1 heterogeneity: variable binding of monoclonal IgG1 antibodies to protein A-sepharose. Mol Immunol. 1984 Oct;21(10):993–998. doi: 10.1016/0161-5890(84)90158-5. [DOI] [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]


