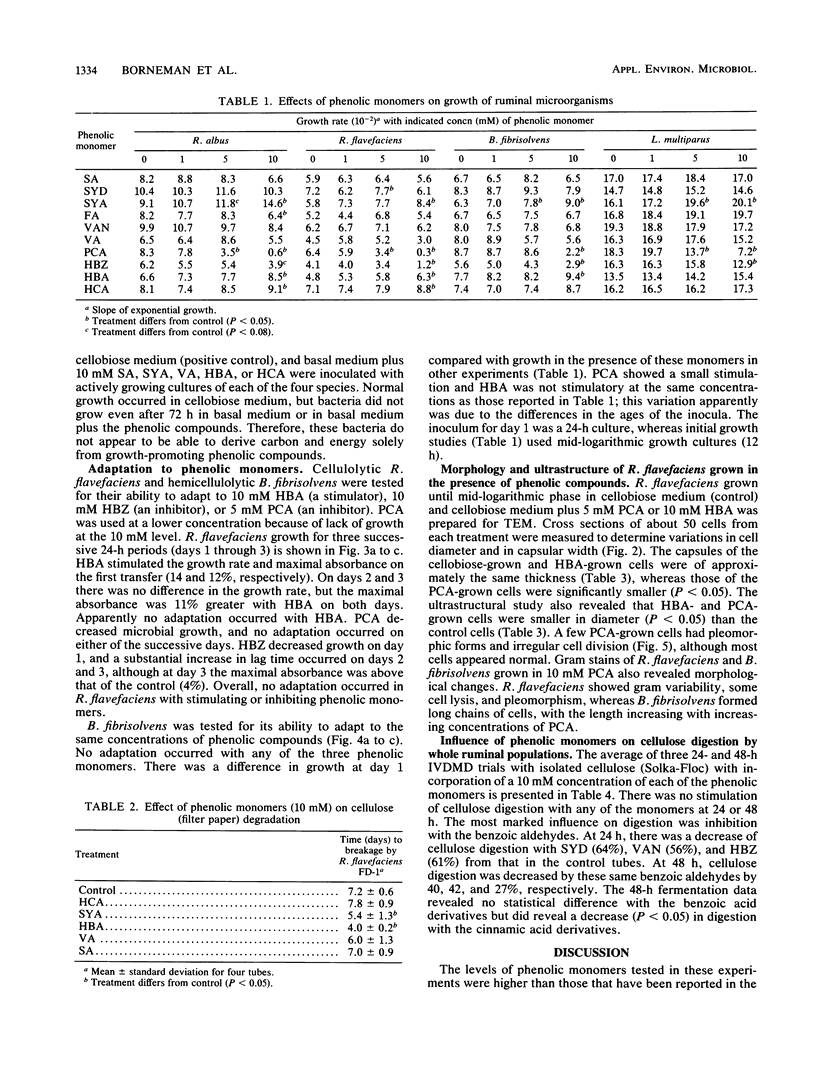
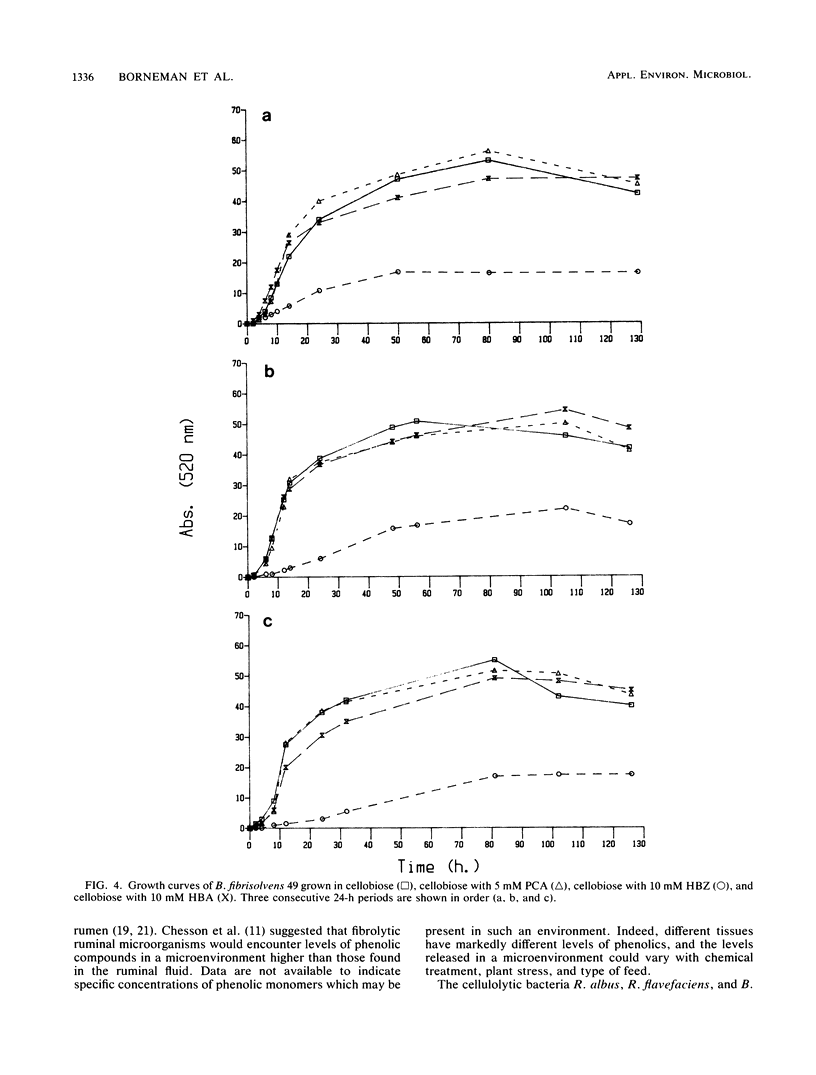
Abstract
Ruminal bacteria were subjected to a series of phenolic compounds in various concentrations to acquire fundamental information on the influence on growth and the potential limits to forage utilization by phenolic monomers. Ruminococcus albus 7, Ruminococcus flavefaciens FD-1, Butyrivibrio fibrisolvens 49, and Lachnospira multiparus D-32 were tested against 1, 5, and 10 mM concentrations of sinapic acid, syringaldehyde, syringic acid, ferulic acid, vanillin, vanillic acid, p-coumaric acid, p-hydroxybenzaldehyde, p-hydroxybenzoic acid, and hydrocinnamic acid. Responses were variable and dependent on the phenolic compound and microbial species. Compounds especially toxic (i.e., resulting in poor growth, effect on several species, dose-related response) were p-coumaric acid and p-hydroxybenzaldehyde, and adaptation to the toxins did not occur after three 24-h periods. Syringic, p-hydroxybenzoic, and hydrocinnamic acids stimulated growth of all four species and also stimulated filter paper degradation by R. flavefaciens. None of the stimulatory compounds supported microbial growth in the absence of carbohydrates. In vitro dry matter digestibility of cellulose (Solka-Floc) was not stimulated by any of the phenolic compounds (10 mM), but the cinnamic acids and benzoic aldehydes (10 mM) reduced (P less than 0.05) digestion by the mixed population in ruminal fluid. Growth of R. flavefaciens in the presence of p-hydroxybenzoic acid (10 mM) or p-coumaric acid (5 mM) resulted in recognizable alterations in cell ultrastructure. Both phenolics caused a reduction in cell size (P less than 0.05), and p-coumaric acid caused a reduction in capsular size (P less than 0.05) and produced occasional pleomorphic cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E. Attack on lignified grass cell walls by a facultatively anaerobic bacterium. Appl Environ Microbiol. 1980 Oct;40(4):809–820. doi: 10.1128/aem.40.4.809-820.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E., Barton F. E., 2nd Rumen microbial attachment and degradation of plant cell walls. Fed Proc. 1983 Jan;42(1):114–121. [PubMed] [Google Scholar]
- Akin D. E. Evaluation by electron microscopy and anaerobic culture of types of rumen bacteria associated with digestion of forage cell walls. Appl Environ Microbiol. 1980 Jan;39(1):242–252. doi: 10.1128/aem.39.1.242-252.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin D. E. Ultrastructure of rumen bacterial attachment to forage cell walls. Appl Environ Microbiol. 1976 Apr;31(4):562–568. doi: 10.1128/aem.31.4.562-568.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Ohmiya K., Shimizu S., Kawakami H. Degradation of dehydrodivanillin by anaerobic bacteria from cow rumen fluid. Appl Environ Microbiol. 1985 Jan;49(1):211–216. doi: 10.1128/aem.49.1.211-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesson A., Stewart C. S., Wallace R. J. Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria. Appl Environ Microbiol. 1982 Sep;44(3):597–603. doi: 10.1128/aem.44.3.597-603.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y., Reinhard M. Methanogenic decomposition of ferulic Acid, a model lignin derivative. Appl Environ Microbiol. 1980 Feb;39(2):436–444. doi: 10.1128/aem.39.2.436-444.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E., Stack R. J. Phenylpropanoic Acid: Growth Factor for Ruminococcus albus. Appl Environ Microbiol. 1982 Jul;44(1):79–83. doi: 10.1128/aem.44.1.79-83.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. J., Fahey G. C., Jr, Merchen N. R. Effects of ruminant digestion and metabolism on phenolic monomers of forages. Br J Nutr. 1983 Nov;50(3):637–651. doi: 10.1079/bjn19830135. [DOI] [PubMed] [Google Scholar]
- Jurd L., Stevens K. L., King A. D., Jr, Mihara K. Antimicrobial properties of natural phenols and related compounds. II. Cinnamylated phenols and their hydrogenation products. J Pharm Sci. 1971 Nov;60(11):1753–1755. doi: 10.1002/jps.2600601145. [DOI] [PubMed] [Google Scholar]
- Leatherwood J. M. Cellulose degradation by Ruminococcus. Fed Proc. 1973 Jul;32(7):1814–1818. [PubMed] [Google Scholar]
- Martin A. K. The origin of urinary aromatic compounds excreted by ruminants. 2. The metabolism of phenolic cinnamic acids to benzoic acid. Br J Nutr. 1982 Jan;47(1):155–164. doi: 10.1079/bjn19820020. [DOI] [PubMed] [Google Scholar]
- McDougall E. I. Studies on ruminant saliva. 1. The composition and output of sheep's saliva. Biochem J. 1948;43(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- Morrison I. M. Structural invesiigations on the lignin-carbohydrate complexes of Lolium perenne. Biochem J. 1974 Apr;139(1):197–204. doi: 10.1042/bj1390197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson H., Irvin R., Costerton J. W., Cheng K. J. Ultrastructure and adhesion properties of Ruminococcus albus. J Bacteriol. 1975 Apr;122(1):278–287. doi: 10.1128/jb.122.1.278-287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stack R. J., Cotta M. A. Effect of 3-phenylpropanoic Acid on growth of and cellulose utilization by cellulolytic ruminal bacteria. Appl Environ Microbiol. 1986 Jul;52(1):209–210. doi: 10.1128/aem.52.1.209-210.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack R. J., Hungate R. E. Effect of 3-Phenylpropanoic Acid on Capsule and Cellulases of Ruminococcus albus 8. Appl Environ Microbiol. 1984 Jul;48(1):218–223. doi: 10.1128/aem.48.1.218-223.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varel V. H., Jung H. J. Influence of forage phenolics on ruminal fibrolytic bacteria and in vitro fiber degradation. Appl Environ Microbiol. 1986 Aug;52(2):275–280. doi: 10.1128/aem.52.2.275-280.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemek J., Kosíková B., Augustín J., Joniak D. Antibiotic properties of lignin components. Folia Microbiol (Praha) 1979;24(6):483–486. doi: 10.1007/BF02927180. [DOI] [PubMed] [Google Scholar]





