Abstract
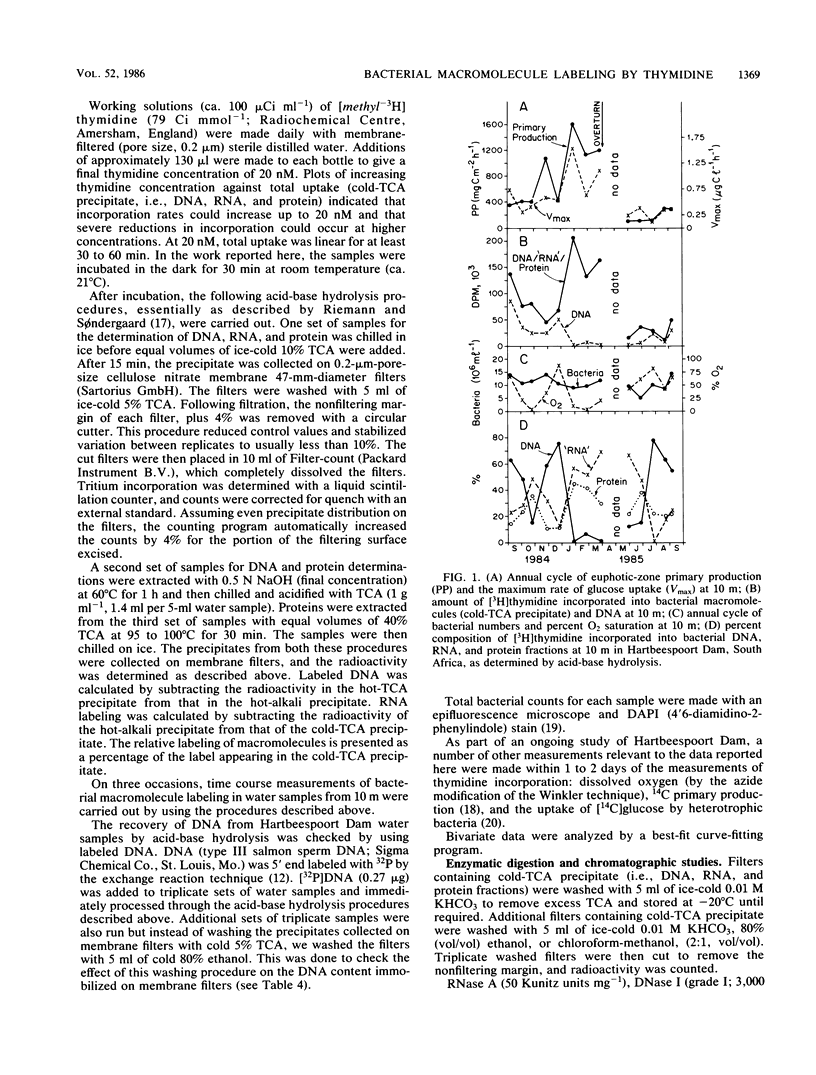
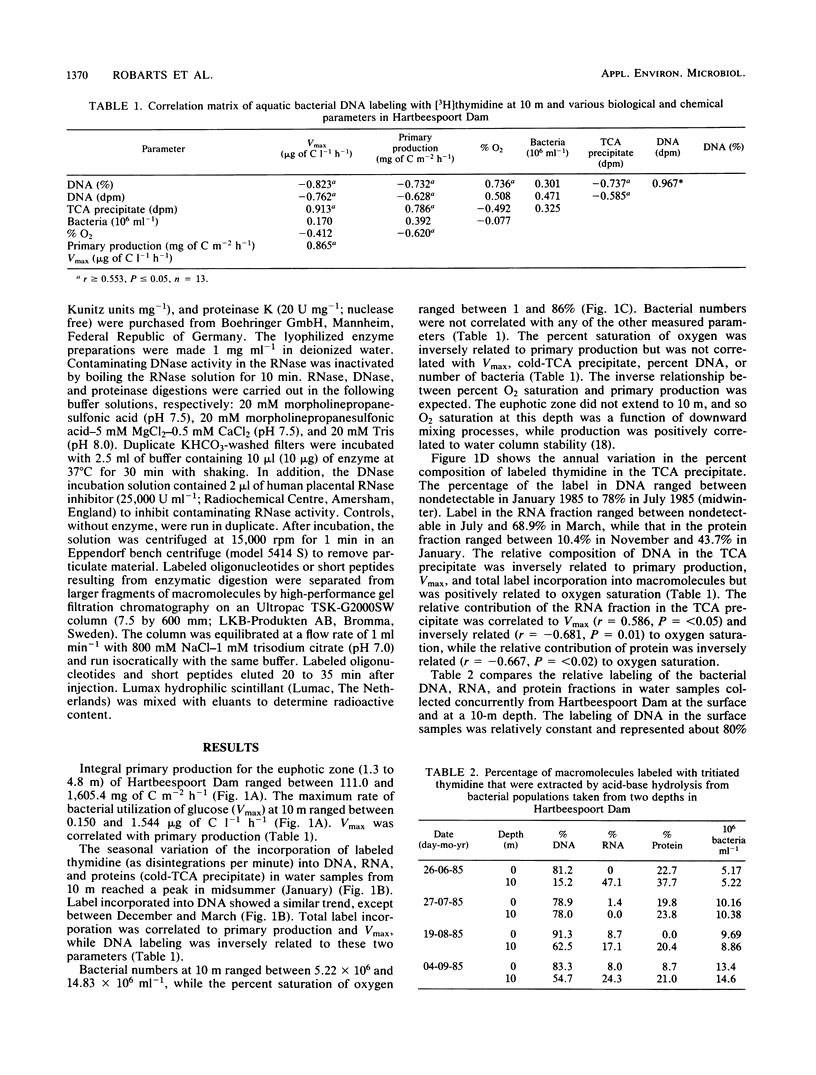
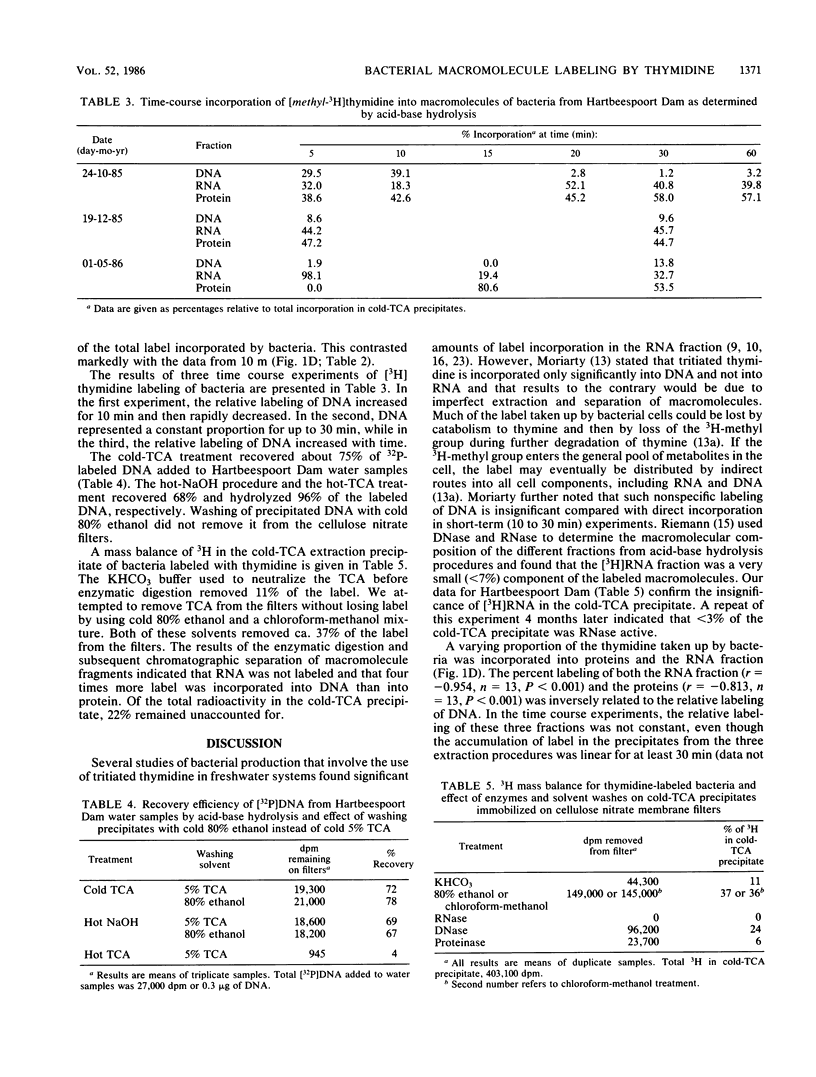
The incorporation of [methyl-3H]thymidine into three macromolecular fractions, designated as DNA, RNA, and protein, by bacteria from Hartbeespoort Dam, South Africa, was measured over 1 year by acid-base hydrolysis procedures. Samples were collected at 10 m, which was at least 5 m beneath the euphotic zone. On four occasions, samples were concurrently collected at the surface. Approximately 80% of the label was incorporated into bacterial DNA in surface samples. At 10 m, total incorporation of label into bacterial macromolecules was correlated to bacterial utilization of glucose (r = 0.913, n = 13, P < 0.001). The labeling of DNA, which ranged between 0 and 78% of total macromolecule incorporation, was inversely related to glucose uptake (r = -0.823), total thymidine incorporation (r = -0.737), and euphotic zone algal production (r = -0.732, n = 13, P < 0.005). With decreased DNA labeling, increasing proportions of label were found in the RNA fraction and proteins. Enzymatic digestion followed by chromatographic separation of macromolecule fragments indicated that DNA and proteins were labeled while RNA was not. The RNA fraction may represent labeled lipids or other macromolecules or both. The data demonstrated a close coupling between phytoplankton production and heterotrophic bacterial activity in this hypertrophic lake but also confirmed the need for the routine extraction and purification of DNA during [methyl-3H]thymidine studies of aquatic bacterial production.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. T., Ahlgren G. M., Ahlgren I. Estimating Bacterioplankton Production by Measuring [H]thymidine Incorporation in a Eutrophic Swedish Lake. Appl Environ Microbiol. 1983 Jun;45(6):1709–1721. doi: 10.1128/aem.45.6.1709-1721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. T., Kuparinen J. Assessing phytoplankton and bacterioplankton production during early spring in lake erken, sweden. Appl Environ Microbiol. 1984 Dec;48(6):1221–1230. doi: 10.1128/aem.48.6.1221-1230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern L. Autoradiographic Studies of [methyl-H]Thymidine Incorporation in a Cyanobacterium (Microcystis wesenbergii)-Bacterium Association and in Selected Algae and Bacteria. Appl Environ Microbiol. 1985 Jan;49(1):232–233. doi: 10.1128/aem.49.1.232-233.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer E., Graf F., Carli S. Rhythmusstörungen und Symptome Während Langzeit-EKG bei ambulanten Patienten. Schweiz Rundsch Med Prax. 1984 Jan 17;73(3):59–65. [PubMed] [Google Scholar]
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl D. M. Selected nucleic Acid precursors in studies of aquatic microbial ecology. Appl Environ Microbiol. 1982 Oct;44(4):891–902. doi: 10.1128/aem.44.4.891-902.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Primary and bacterial production in two dimictic indiana lakes. Appl Environ Microbiol. 1985 Mar;49(3):485–491. doi: 10.1128/aem.49.3.485-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell C. R., Konopka A. Seasonal bacterial production in a dimictic lake as measured by increases in cell numbers and thymidine incorporation. Appl Environ Microbiol. 1985 Mar;49(3):492–500. doi: 10.1128/aem.49.3.492-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Pollard P. C., Moriarty D. J. Validity of the tritiated thymidine method for estimating bacterial growth rates: measurement of isotope dilution during DNA synthesis. Appl Environ Microbiol. 1984 Dec;48(6):1076–1083. doi: 10.1128/aem.48.6.1076-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann B., Søndergaard M. Measurements of diel rates of bacterial secondary production in aquatic environments. Appl Environ Microbiol. 1984 Apr;47(4):632–638. doi: 10.1128/aem.47.4.632-638.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]



