Abstract
Yeast two-hybrid and genetic interaction screens indicate that Bir1p, a yeast protein containing phylogenetically conserved antiapoptotic repeat domains called baculovirus inhibitor of apoptosis repeats (BIRs), is involved in chromosome segregation events. In the two-hybrid screen, Bir1p specifically interacts with Ndc10p, an essential component of the yeast kinetochore. Although Bir1p carries two BIR motifs in the N-terminal region, the C-terminal third of the protein is sufficient to provide strong interaction with Ndc10p and moderate interaction with Skp1p, another essential component of the yeast kinetochore. In addition, deletion of BIR1 is synthetically lethal with deletion of CBF1 or CTF19, genes specifying two other components of the yeast kinetochore. Yeast cells deleted of BIR1 have a chromosome-loss phenotype, which can be completely rescued by elevating NDC10 dosage. Furthermore, overexpression of either full-length or the C-terminal region of Bir1p can efficiently suppress the chromosome-loss phenotype of both bir1Δ null and skp1-4 mutants. Our data suggest that Bir1p participates in chromosome segregation events, either directly or via interaction with kinetochore proteins, and these effects are apparently not mediated by the BIR domains of Bir1p.
The yeast (Saccharomyces cerevisiae) BIR1 gene specifies a member of the baculovirus inhibitor of apoptosis repeats (BIR)-containing proteins that are found in viruses, yeast, and metazoans (1). Most mammalian and insect BIR-proteins inhibit cell death when apoptosis is induced by a variety of agents (for recent reviews, see refs. 2 and 3). Human survivin, a particularly interesting example, is abundantly expressed in cancer cells and specifically associates with the mitotic spindle (4). Disruption of the survivin–microtubule interaction results in loss of its antiapoptosis function. Caenorhabditis elegans BIR-1 is probably not involved in the general regulation of apoptosis but is required for embryonic cytokinesis (5). Survivin might be functionally similar to the nematode BIR-1, because survivin can partially substitute for BIR-1 in cytokinesis. The yeasts S. cerevisiae and Schizosaccharomyces pombe each contain single BIR proteins that seem to be involved in some as yet unknown aspect of cell division (6).
One important participant in the cell division process is the centromere kinetochore, a macromolecular structure responsible for accurate chromosome segregation. Budding yeasts, such as S. cerevisiae, contain so-called “point” centromeres. In contrast to the large more complex “regional” centromeres found in higher eukaryotes, the minimum DNA sequence required for faithful segregation of the yeast chromosomes consists of only 125 bp (the CEN locus), containing three conserved DNA elements, CDEI, CDEII, and CDEIII (reviewed in ref. 7). However, the yeast kinetochore seems to be relatively complex, with 11 protein components identified to date (8, 9). The core centromeric protein complex in yeast (CBF3) consists of four essential subunits: Ndc10p (110 kDa), Cep3p (64 kDa), Ctf13p (58 kDa), and Skp1p (24 kDa; ref. 7). This core complex is thought to be present at the CEN locus throughout the yeast cell cycle and serves as a foundation for assembly of a multicomponent complex that interacts with the microtubules during mitosis and meiosis. Herein, we report that Bir1p is involved in the chromosome segregation process and interacts physically and genetically with several components of the yeast kinetochore. These results extend and confirm previously postulated roles for the BIR proteins in cell division (4, 6).
Materials and Methods
Yeast Strains and Cell Culture.
The S. cerevisiae strains used in this study are listed in Table 1. The diploid strain YHY399 was constructed by mating haploid strains YPH278 and YPH1015 and subsequently plating on 5-fluoroorotic acid plates to identify colonies that lost CFIII [(CEN3. L. YPH278) URA3 SUP11] but retained CFIII [(CEN3. L. YPH983) HIS3 SUP11]. Yeast cultures were grown in yeast extract/peptone/dextrose or, to maintain selection for plasmids, in synthetic minimal medium (SD) supplemented with the appropriate amino acids and/or bases. For tetrad dissection, diploid cells were streaked on sporulation plates and incubated at 25°C for 7–10 days. Media for yeast growth and sporulation have been described (10). For the colony color sectoring assay (11), adenine was added to growth media at 6 mg/liter. Yeast transformations were performed by the lithium acetate method (12).
Table 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| YPH278 | MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 ura3-52 CFIII (CEN3.L. YPH278) URA3 SUPP11 | P. Hieter* |
| YPH1015 | MATa ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 CFIII (CEN3.L. YPH983) HIS3 SUPP11 | P. Hieter |
| YPH1161 | MATa ade2-101 his3-Δ200 leu2-Δ1 lys 2-801 trp1-Δ63 ura3-52 skp1-Δ::TRP1 skp1-4::LEU2 | P. Hieter |
| CFIII (CEN3.L. YPH983) HIS3 SUP11 | ||
| YPH1317 | MATa ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 ctf19-Δ1::TRP1 | P. Hieter |
| s30 | MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 ura3-52 ctf13-30 CFIII (CEN3.L. YPH278) URA3 SUPP11 | P. Hieter |
| YHY399 | MATa/Matα ade2-101/ade2-101 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 lys-2-801/lys2-801 | This work |
| TRP1/trp1-Δ63 ura3-52/ura3-52 CFIII (CEN3.L. YPH983) HIS3 SUPP11 | ||
| YHY 400 | MATa/MATα ade2-101/ade2-101 his3-Δ200/his3-Δ200 leu2-Δ1/leu2-Δ1 lys2-801/lys2-801 | This work |
| TRP1/trp1-Δ63 ura3-52/ura3-52 BIR1/bir1Δ::LEU2 CFIII (CEN3.L. YPH983) HIS3 SUPP11 | ||
| YHY401 | MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 CFIII (CEN3.L. YPH983) HIS3 SUP11 | This work |
| YHY402 | MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 bir1Δ::LEU2 | This work |
| CFIII (CEN3.L. YPH983) HIS3 SUP11 | ||
| YHY403 | MATa ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 bir1Δ::LEU2 | This work |
| CFIII (CEN3.L. YPH983) HIS3 SUPP11 | ||
| YHY13 | MATa ade2-101 his3-Δ200 leu2-3,112 lys2-801 trp1-Δ63 ura3-52 ndc10-1 | H. Yoon |
| YJL146 | Mata ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 cyh2R mcm21Δ::TRP1 | J. Lechner† |
| YJL158 | MATa ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 cyh2R okp1-5::TRP1 | J. Lechner |
| YSS90 | MATa ade2-101 leu2-Δ1 lys2-801 ura3-52 cbf1Δ::URA3 | W. Jiang‡ |
| 1cAS281 | Mata ade2 his3 leu2 lys2 trp1 ura3 cep3-1 | D. Koshland§ |
| PM1002-4c | MATa ade2 ade3 leu2 ura3 mif2-3 | D. Koshland |
| CSE4-1 (3a) | MATα ade2 his3 leu2 trp1 ura3 cse4-1 | M. Fitzgerald-Hayes¶ |
| WSCF101 | MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 trp1-Δ63 ura3-52 slk19Δ::HIS3 | I. Fitch∥ |
| CFIII (CEN3.L. YPH278) URA3 SUP11 |
*Center for Molecular Medicine and Therapeutics, University of British Columbia, Vancouver, BC V5Z4H4, Canada.
†BZH, Ruprecht-Karls-Universität Heidelberg, 69120 Heidelberg, Germany.
This laboratory.
§ Carnegie Institute of Washington, Baltimore, MD 21210.
¶ Biochemistry and Molecular Biology Department, University of Massachusetts, Amherst, MA 01003.
∥This laboratory.
Identification of BIR1 by Two-Hybrid Interaction with Ndc10p.
The C-terminal 850-amino acid region of Ndc10p fused to the Gal4 DNA-binding domain (DBD) was used as bait to screen yeast two-hybrid genomic libraries (13). Among the ≈1 × 106 transformants screened, 24 clones showed reproducible interaction with Ndc10p. The subsequent analysis of the genomic inserts revealed that five clones carried ORFs fused in frame with the Gal4 activation domain (ACD). One plasmid, pACD-Bir1 (C332), contained the C-terminal 332 amino acids of Bir1p fused to ACD. The full-length BIR1 gene and flanking sequences were isolated from a yeast genomic library constructed in the CEN plasmid vector YCp50 (purchased from the American Type Culture Collection; ref. 14). The 4.0-kilobase (kb) SnaBI fragment carrying BIR1 and flanking sequences was introduced into the multicopy vector YEp24 (2 μm, URA3) to obtain YEp24-BIR1. The entire BIR1 coding region was subcloned into the EcoRI and SmaI sites of the pGBD-C3 vector (13) to construct a fusion between DBD and Bir1p. The resulting plasmid was termed pDBD-Bir1 (full).
Plasmids.
Plasmids carrying either DBD-fused or ACD-fused CBF1 were made as follows. The CBF1 ORF was cloned by PCR with yeast genomic DNA and EcoRI-linked and BglII-linked oligonucleotides (5′-CAGAATTCTTAACGATGAACTCTCTG-3′ and 5′-TTAGATCTCAAGCCTCATGTGGATTA-3′). The PCR product was cut with EcoRI and BglII and subcloned into the EcoRI and BglII sites of pGBD-C3 or pGAD-C3. Plasmids carrying either DBD-fused or ACD-fused Ndc10p, Cep3p, Ctf13p, and Skp1p were provided by J. Lechner. The construction of plasmids carrying truncated Bir1p fragments is described below. The plasmid pDBD-Bir1 (full) was cut with ClaI and religated to remove the 3′ 2.2-kb ClaI fragment of BIR1. The resulting plasmid pDBD-Bir1 (N245) carries the N-terminal 245 amino acids of Bir1p fused to DBD. Plasmids pDBD-Bir1 (N552) and pDBD-Bir1 (N659) were constructed by removing the 3′ 1.3-kb BamHI and 3′ 1.0-kb BglII fragments, respectively, from pDBD-Bir1 (full). The 3′ 1.3-kb BamHI fragment was subcloned into the BamHI site of the pGBD-C1 vector to obtain the plasmid pDBD-Bir1 (C405), which expresses the C-terminal 405 amino acids of Bir1p fused to DBD. Finally, pDBD-Bir1 (C297) was made by inserting the 3′ 1.0-kb BglII fragment from pDBD-Bir1 (C405) into the BamHI site of pGBD-C3. The multicopy plasmids YEp24-NDC10, YEp24-CTF13, and YEp24-SKP1 carry the 6.6-kb BamHI fragment of NDC10 derived from pWJ316-110 (15); the 2.2-kb XbaI fragment of CTF13 originated from pKF11 (obtained from P. Hieter), and the 1.7-kb SpeI–ClaI fragment of SKP1 originated from pRS316-SKP1 (P. Hieter). YEp24-CEP3 is the same as pAS480/1 (from D. Koshland).
Construction of the bir1Δ Null Mutant.
The BIR1 gene disruption was constructed in diploid strain YHY400 by replacing the internal 1.8-kb MscI–BglII fragment of BIR1 with a 2.0-kb HpaI–SalI fragment containing yeast LEU2. The BglII and SalI sites were converted to blunt ends with T4 DNA polymerase before ligation. The resulting diploid strain bearing the minichromosome CFIII (CEN3.L. YPH983) HIS3 SUP11 and one disrupted copy of BIR1 was sporulated, and the tetrads were analyzed. Among 30 tetrads, 29 showed four viable spores and 2+:2− segregation of the LEU2 marker. The bir1 disruption was verified by genomic Southern blot hybridization with DNA of the haploid strains.
Results
BIR1 Interacts with Essential Kinetochore Protein Ndc10p.
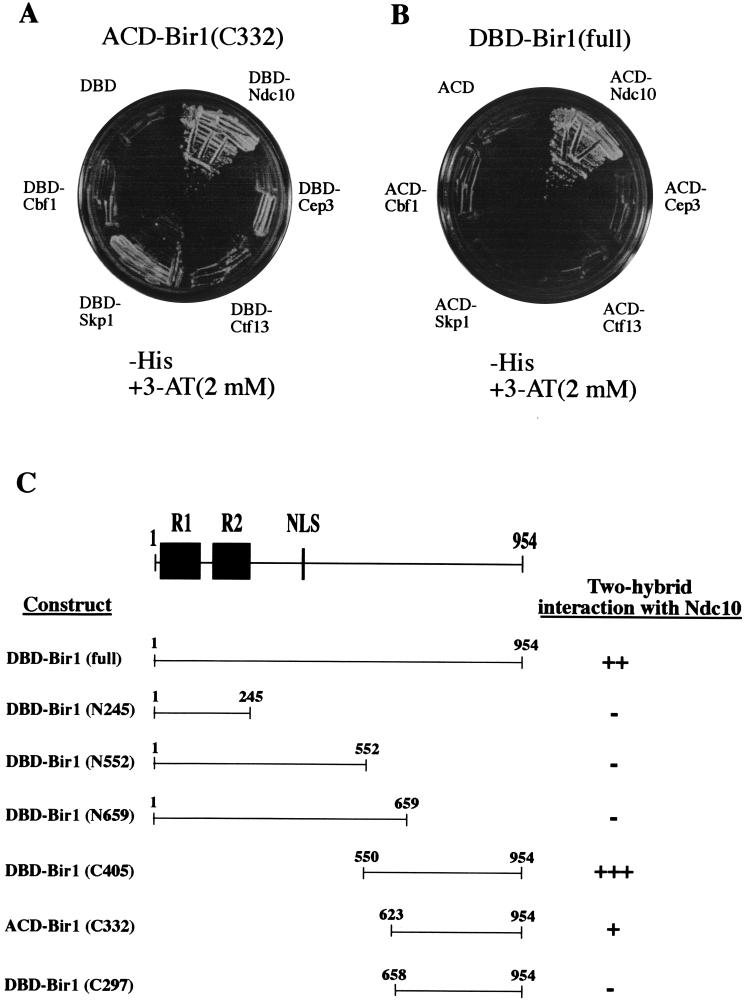
In experiments directed toward the isolation of unknown components of the yeast centromere kinetochore, we performed an extensive two-hybrid screen by using Ndc10p fused to the Gal4 DNA-binding protein as bait. A strongly positive clone isolated multiple times in this screen was shown to contain the C-terminal third of the BIR1 gene product fused to ACD (C332; Fig. 1A). This C-terminal region of Bir1p was tested further for two-hybrid interaction with the other CBF3 components—Cep3p, Ctf13p, and Skp1p—and with Cbf1p (16–23). C332 showed moderate interaction with Skp1p and perhaps very weak interaction with Ctf13p and Cep3p (Fig. 1A). This C-terminal region of Bir1p had no binding activity toward other known kinetochore proteins, including Mif2p, Cse4p, Ctf19p, Mcm21p, Okp1p, and Slk19p (8, 9, 24–26). Full-length (954 amino acids) BIR1-DBD interacted only with Ndc10p among the 11 kinetochore proteins (Fig. 1B). To determine the regions of Bir1p interacting with Ndc10p, we generated a series of truncated Bir1p-DBD fusions and tested them for Ndc10p-binding activity in the two-hybrid system. The N-terminal regions bearing the BIR domains (N245, N552, and N659) showed no binding activity. Although the C-terminal 332 amino acids (C332) were sufficient to provide strong interaction with Ndc10p (Fig. 1C), the strongest binding activity was observed with the C-terminal 405 amino acids (C405; Fig. 1C).
Figure 1.
In vivo binding of Bir1p to Ndc10p. (A) Two-hybrid interaction between a Bir1p fragment and known kinetochore proteins. A tester strain PJ69–4A (17) carrying the plasmid pACD-Bir1 (C332) was transformed with various plasmids that express DBD alone or DBD-fused Ndc10p, Cep3p, Ctf13p, Skp1p, or Cbf1p. The transformants were streaked on SD/His− medium containing 2 mM 3-AT (3-amino-1 2,4-triazole) to test expression of the HIS3 reporter gene. (B) Two-hybrid assay with strain PJ69-4A carrying pDBD-Bir1 (full). Strain PJ69-4A/pDBD-Bir1 (full) bearing plasmids expressing ACD-fused Mif2p, Cse4p, Ctf19p, Mcm21p, Okp1p, or Slk19p did not grow on minimal salt-dextrose/His− medium containing 2 mM 3-AT (not shown). (C) Identification of Bir1p regions interacting with Ndc10p. (Top) Schematic view of Bir1p showing the relative locations of BIR domains 1 (R1), 2 (R2), and of nuclear localization signal (NLS), a bipartite nuclear localization signal. Various truncated Bir1p fragments as shown here (See Materials and Methods) were tested for two-hybrid interactions with Ndc10p on SD/His− medium containing 2 mM 3-AT.
The bir1Δ Null Mutant Has a Chromosome Missegregation Phenotype.
Deletion of BIR1 in haploid yeast did not cause noticeable defects under the various growth conditions that we tested. The growth of the bir1Δ strain was similar to that of the isogenic BIR1 wild type when subjected to different growth temperatures, carbon sources, metal ions, and mitotic poisons. The spindle and nuclear morphology of bir1Δ null cells also seemed normal. However, bir1Δ null cells had a chromosome missegregation phenotype, as determined by an accurate and highly reproducible colony color sectoring assay (Table 2). Disruption of BIR1 led to a 5-fold increase in the marked chromosome fragment loss rate per cell division (Table 2). In addition, bir1Δ null cultures grown in media selective for the chromosome fragment contained ≈20 times more segregants lacking the tester chromosome than did the isogenic wild-type cells (Table 2, red colonies).
Table 2.
Rates of minichromosome loss in bir1Δ cells
| Genotype | Red colonies | Half-sectored colonies* | Total colonies | Loss rate/cell division† | Fold increase‡ |
|---|---|---|---|---|---|
| BIR1 | 6 | 6 | 5,409 | 1.11 × 10−3 | 1.0 |
| bir1Δ | 134 | 27 | 4,878 | 5.69 × 10−3 | 5.1 |
The colony color sectoring assay was performed with isogenic wild-type BIR1 (YHY401) and bir1Δ (YHY402) strains. A single colony from each strain was grown overnight in SD/His− medium and plated for single colonies on yeast extract/peptone/dextrose.
Includes colonies that contain at least a full half sector. The mother cell underwent a chromosome loss or missegregation event in the first division.
† Loss rate/cell division = half-sectored colonies/(total colonies − red colonies).
‡ Fold increase = loss rate/cell division of bir1Δ cells divided by loss rate/cell division of BIR1 cells.
Bir1Δ Is Synthetically Lethal with Mutations in the Kinetochore Protein Genes cbf1 and ctf19.
To explore a potential role of Bir1p in kinetochore function, genetic analysis was used to look for synthetic phenotypes revealed by strains carrying bir1Δ and mutations in known kinetochore protein genes (Table 3). Crosses were made between a bir1Δ null strain and strains bearing mutations in each of the 11 different known kinetochore proteins. Double mutants were isolated after sporulation and tetrad dissection of the resulting heterozygous diploids. We were unable to isolate a bir1Δ skp1-4 double mutant because of a severe sporulation defect in the corresponding heterozygous diploid. Among the remaining 10 kinetochore protein mutants, only cbf1Δ and ctf19Δ had synthetic lethal interactions with the bir1Δ null (Table 3). Although cells lacking either CBF1 or CTF19 alone are viable, the bir1Δ cbf1Δ and bir1Δ ctf19Δ double-mutant strains could not be found among spores germinated at 25°C. Cbf1p and Ctf19p are not absolutely essential for centromere function, but mutations in these genes result in significantly increased chromosome missegregation frequencies (8, 21–23, 26). We also looked for genetic interactions between bir1Δ and spindle checkpoint mutants. No synthetically lethal interactions were observed between bir1Δ and mad1, mad2, mad3, bub1, bub2, and bub3 null mutants (data not shown).
Table 3.
Synthetic lethality studies with bir1Δ and various kinetochore mutants
| Double-mutant genotype | Phenotype | Total no. of tetrads |
|---|---|---|
| bir1Δndc10-1 | Viable | 29 |
| bir1Δcep3-1 | Viable | 29 |
| bir1Δctf13-30 | Viable | 34 |
| bir1Δcbf1Δ | SL | 65 |
| bir1Δctf19Δ | SL | 16 |
| bir1Δmcm21Δ | Viable | 36 |
| bir1Δokp1-5 | Viable | 29 |
| bir1Δmif2-3 | Viable | 31 |
| bir1Δcse4-1 | Viable | 33 |
| bir1Δslk19Δ | Viable | 39 |
SL, synthetic lethality. Heterozygous diploids containing bir1Δ and the various kinetochore protein mutations listed above were sporulated; tetrads were dissected; and the haploid progeny were scored for presence of the double mutations. SL indicates no viable double mutants found in the number of tetrads listed above.
Overexpression of NDC10 Reverses the Chromosome-Loss Phenotype of bir1Δ Cells.
Direct or indirect in vivo interactions between proteins often can be revealed by overexpressing a given wild-type gene in various mutant cells and looking for pleiotropic effects on characteristic mutant phenotype(s). We therefore examined the effects of increasing the dosage of known kinetochore genes on the chromosome-loss phenotype seen in the bir1Δ null mutant. As shown in Table 4, overexpression of NDC10 completely rescued the chromosome-loss phenotype of bir1Δ cells. However, overexpressing any of the genes specifying the other three CBF3 subunits (CEP3, CTF13, and SKP1) produced no dosage effects on the bir1Δ mutant (Table 4). As a control, overexpression of full-length BIR1 was shown also to suppress the bir1Δ chromosome-loss phenotype. Surprisingly, it was found that overexpression of the 3′ BIR1 fragment that produces the C-terminal 405 amino acids (C405) lacking the BIR motifs also stabilized the tester minichromosome in bir1Δ null cells (Table 4). However, elevated levels of the 5′ BIR1 fragment that expresses the N-terminal 552 amino acids including both BIR motifs (N552) had little or no effect (Table 4), indicating that the BIR motifs are not significantly involved in Bir1p functions related to chromosome segregation. Overexpressing full-length BIR1, N552, or C405 had no effects on minichromosome stability in isogenic wild-type cells.
Table 4.
Dosage effects of kinetochore protein genes on the bir1Δ chromosome-loss phenotype
| Plasmid | Red colonies | Half-sectored colonies | Total colonies | Loss rate/ cell division* | Fold decrease† |
|---|---|---|---|---|---|
| YEp24 | 48 | 19 | 4,794 | 4.00 × 10−3 | 1.0 |
| YEp24-BIR1 | 3 | 1 | 13,422 | 7.45 × 10−5 | 53.7 |
| YEp24-BIR1 (N552) | 9 | 4 | 2,848 | 1.41 × 10−3 | 2.8 |
| YEp24-BIR1 (C405) | 3 | 1 | 14,108 | 7.09 × 10−5 | 56.5 |
| YEp24-NDC10 | 0 | 1 | 13,540 | 7.39 × 10−5 | 54.2 |
| YEp24-CEP3 | 75 | 3 | 2,955 | 1.04 × 10−3 | 3.8 |
| YEP24-CTF13 | 24 | 6 | 5,200 | 1.16 × 10−3 | 3.5 |
| YEP24-SKP1 | 24 | 12 | 2,279 | 5.32 × 10−3 | 0.8 |
The bir1Δ mutant (YHY402) was transformed with a high-copy plasmid YEp24 (2 μm, URA3) or YEp24 carrying BIR1, NDC10, CEP3, CTF13, or SKP1. In addition to the complete BIR1 ORF, a 1.7-kb fragment that expresses the N-terminal 552 amino acids of Bir1p or a 1.2-kb fragment that expreses the C-terminal 405 amino acids of Bir1p was also subcloned into YEp24 and tested for the ability to complement the bir1 null mutation. The colony color sectoring assay was performed as described in Table 2.
Loss rate/cell division = half-sectored colonies/(total colonies − red colonies).
† Fold decrease = loss rate/cell division of bir1Δ cells carrying YEp24 divided by loss rate/cell division of bir1Δ cells containing each of the indicated plasmids.
High Levels of BIR1 Increase Chromosome Segregation Fidelity in skp1-4 Cells.
The reciprocal dosage suppression experiment was performed by overexpressing BIR1 in various kinetochore mutant strains. The temperature-sensitive skp1-4 mutant has a pronounced chromosome segregation defect even at the permissive temperature, 25°C. We observed that high levels of BIR1 fully rescued the severe chromosome-loss phenotype of the skp1-4 mutant at 25°C (Table 5). Furthermore, overexpressing the 3′ BIR1 fragment (C405) was as effective as a single gene dosage of SKP1 (pRS316-SKP1) in rescuing tester chromosome stability in skp1-4 cells. Again, the 5′ BIR1 fragment (N552) was unable to rescue the skp1-4 chromosome-loss phenotype (Table 5). However, the temperature-sensitive growth phenotype of skp1-4 cells was not suppressed by overexpression of BIR1. In fact, BIR1 is not a dosage suppressor of the growth defects seen in any of the known temperature-sensitive kinetochore mutants (data not shown).
Table 5.
Dosage effects of BIR1 expression on the skp1-4 chromosome-loss phenotype
| Plasmid | Red colonies | Half-sectored colonies | Total colonies | Loss rate/ cell division* | Fold decrease† |
|---|---|---|---|---|---|
| YEp24 | 79 | 55 | 2,418 | 2.35 × 10−2 | 1.0 |
| YEp24-SKP1 | 0 | 1 | 3,577 | 2.80 × 10−4 | 84.1 |
| YEp24-CTF13 | 2 | 1 | 3,567 | 2.81 × 10−4 | 83.8 |
| YEp24-NDC10 | 66 | 36 | 2,356 | 1.57 × 10−2 | 1.5 |
| YEP24-CEP3 | 65 | 42 | 3,404 | 1.26 × 10−2 | 1.9 |
| YEp24-BIR1 | 0 | 1 | 6,933 | 1.44 × 10−4 | 163.0 |
| YEP24-BIR1 (N552) | 63 | 44 | 2,103 | 2.16 × 10−2 | 1.1 |
| YEP24-BIR1 (C405) | 4 | 4 | 2,349 | 1.71 × 10−3 | 13.8 |
| pRS316-SKP1 | 2 | 4 | 2,641 | 1.52 × 10−3 | 15.5 |
A high-copy plasmid, YEp24, carrying SKP1, CTF13, NDC10, CEP3, BIR1, 5′ 1.7-kb (N552), or 3′ 1.2-kb (C405) fragment of BIR1 was introduced into skp1-4 cells, and the colony sectoring assay was performed as described in Table 2, except that the plates were incubated at 25°C for 5 days. The plasmid pRS316 (CEN6, URA3) was used to obtain single gene dosage of SKP1.
*Loss rate/cell division = half-sectored colonies/(total colonies − red colonies).
† Fold decrease = loss rate/cell division of skp1-4 cells carrying YEp24 divided by loss rate/cell division of skp1-4 cells containing each of the indicated plasmids.
Discussion
Several lines of evidence point to a role for yeast Bir1p in some aspect of the chromosome segregation process, most likely in kinetochore function. Deletion of BIR1 is not lethal but does result in a decrease in chromosome segregation fidelity. The BIR1 gene product interacts in vivo either directly or indirectly with four known kinetochore proteins: Ndc10p, Cbf1p, Ctf19p, and Skp1p. Thus, the combined results of two-hybrid and genetic interaction assays suggest that yeast Bir1p participates in chromosome segregation events either directly or via interaction with known kinetochore proteins. However, we do not yet know whether Bir1p is a structural component of the yeast kinetochore. In vivo cross-linking and centromere chromatin immunoprecipitation studies failed to identify the presence of Bir1p in the kinetochore. This result is not conclusive, however, because proteins buried internally in the chromatin mass might not be accessible to interaction with antibodies.
Uren et al. (6) recently reported that a fusion protein between the N-terminal region of Bir1p (residues 1–474) and green fluorescent protein (GFP) is distributed uniformly through the yeast nucleoplasm, whereas another fusion protein, Bir1p (residues 249–954)-GFP, is associated with a fibrillar structure resembling the mitotic spindle. The latter finding is interesting, because human survivin, another BIR-containing protein, has been localized to the mitotic spindle in mitotic human cells (4). We have made several attempts to localize Bir1p by using full-length Bir1p-GFP and Bir1p-hemagglutinin fusion constructs integrated into the chromosomal BIR1 locus. Neither GFP fluorescence nor indirect immunofluorescence against GFP or hemagglutinin produced a detectable signal, although both fusion proteins were expressed according to Western blot analysis (H.-J.Y. and J.C., unpublished data). Uren et al. (6) also reported that they were unable to localize the plasmid-borne full-length Bir1p-GFP fusion protein.
The presence of the conserved BIR sequence domains in Bir1p suggests a role for this protein in apoptosis. It has been shown previously that apoptosis can be induced in yeast by depletion of glutathione or by low external doses of H2O2 (27). Yeast can also be triggered into apoptosis by a specific mutation (S565G) in CDC48 or by overexpression of mammalian Bax (28–30). In both cases, oxygen radicals accumulate in the cell, and radical depletion or hypoxia prevents apoptosis (27). The yeast genome lacks sequences specifying any components of the established apoptotic death machinery. Bir1p is the only budding yeast protein that belongs to the evolutionarily conserved, cell-death protein family. However, the two BIR domains in Bir1p seem to perform no obvious function in yeast viability, because bir1Δ null cells are viable and grow as well as the isogenic wild-type cells. Deletion of BIR1 becomes essential for cell viability only when combined with disruption of kinetochore protein genes CBF1 or CTF19. The BIR domains also do not seem to play a role in chromosome segregation events. The C-terminal region of Bir1p lacking both BIR domains is sufficient to interact with Ndc10p or Skp1p and to rescue the chromosome-loss phenotype of both bir1Δ null and skp1-4 cells. In addition, our preliminary data suggest that Bir1p is not involved in controlling apoptotic death of cdc48-S565G yeast cells or gsh1Δ cells depleted of glutathione, because deletion or overexpression of BIR1 has no effect on these processes. Thus, the function of the BIR domains in Bir1p is still unclear. Previous studies on the yeast and nematode BIR proteins also suggested that they function in cell division and probably not in antiapoptosis (5, 6). Further functional characterization of yeast Bir1p should aid in unraveling the cellular functions of various BIR proteins.
Acknowledgments
We thank P. James for the yeast two-hybrid strain and libraries and J. Lechner, P. Hieter, D. Koshland, M. Fitzgerald-Hayes, J. Kilmartin, W. Jiang, and I. Fitch for supplying various plasmids and yeast strains. We also thank members of the Carbon and Clarke laboratories for many discussions and comments on the manuscript. D. Tomkiel and E. George deserve special thanks for their technical support. This research was supported by National Institutes of Health Grant CA-11034 (National Cancer Institute). J.C. is an American Cancer Society Research Professor.
Abbreviations
- BIR
baculovirus inhibitor of apoptosis repeats
- SD
synthetic minimal medium
- DBD
DNA-binding domain
- ACD
activation domain
- kb
kilobase
- GFP
green fluorescent protein
References
- 1.Uren A G, Coulson E J, Vaux D L. Trends Biochem Sci. 1998;23:159–162. doi: 10.1016/s0968-0004(98)01198-0. [DOI] [PubMed] [Google Scholar]
- 2.Deveraux Q L, Reed J C. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 3.Miller L K. Trends Cell Biol. 1999;9:323–328. doi: 10.1016/s0962-8924(99)01609-8. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Ambrosini G, Chu E Y, Plescia J, Tognin S, Marchisio P C, Altieri D C. Nature (London) 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 5.Fraser A G, James C, Evan G I, Hengartner M O. Curr Biol. 1999;9:292–301. doi: 10.1016/s0960-9822(99)80137-7. [DOI] [PubMed] [Google Scholar]
- 6.Uren A G, Beilharz T, O’Connell M J, Bugg S J, Driel R, Vaux D L, Lithgow T. Proc Natl Acad Sci USA. 1999;96:10170–10175. doi: 10.1073/pnas.96.18.10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke L. Curr Opin Genet Dev. 1998;8:212–218. doi: 10.1016/s0959-437x(98)80143-3. [DOI] [PubMed] [Google Scholar]
- 8.Ortiz J, Stemmann O, Rank S, Lechner J. Genes Dev. 1999;13:1140–1155. doi: 10.1101/gad.13.9.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X, Kahana J A, Silver P A, Morphew M K, McIntosh J R, Fitch I T, Carbon J, Saunders W S. J Cell Biol. 1999;146:415–425. doi: 10.1083/jcb.146.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman F, Fink G R, Hicks J B. Laboratory Course Manual for Methods in Yeast Genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1995. [Google Scholar]
- 11.Hieter P, Mann C, Snyder M, Davis R W. Cell. 1985;40:381–392. doi: 10.1016/0092-8674(85)90152-7. [DOI] [PubMed] [Google Scholar]
- 12.Ito H, Fukuda Y, Murata K, Kimura A. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James P, Halladay J, Craig E A. Genetics. 1996;144:1425–1436. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose M D, Novick P, Thomas J H, Botstein D, Fink G R. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- 15.Jiang W, Lechner J, Carbon J. J Cell Biol. 1993;121:513–519. doi: 10.1083/jcb.121.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lechner J. EMBO J. 1994;13:5203–5211. doi: 10.1002/j.1460-2075.1994.tb06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strunnikov A V, Kingsbury J, Koshland D. J Cell Biol. 1995;128:749–760. doi: 10.1083/jcb.128.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doheny K F, Sorger P K, Hyman A A, Tugendreich S, Spencer F, Hieter P. Cell. 1993;73:761–774. doi: 10.1016/0092-8674(93)90255-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connelly C, Hieter P. Cell. 1996;86:275–285. doi: 10.1016/S0092-8674(00)80099-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bram R J, Kornberg R D. Mol Cell Biol. 1987;7:403–409. doi: 10.1128/mcb.7.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker R E, Masison D C. Mol Cell Biol. 1990;10:2458–2467. doi: 10.1128/mcb.10.6.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai M, Davis R W. Cell. 1990;61:437–446. doi: 10.1016/0092-8674(90)90525-j. [DOI] [PubMed] [Google Scholar]
- 23.Mellor J, Jiang W, Funk M, Rathjen J, Barnes C A, Hinz T, Hegemann J H, Philippsen P. EMBO J. 1990;9:4017–4026. doi: 10.1002/j.1460-2075.1990.tb07623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meluh P B, Koshland D. Mol Biol Cell. 1995;6:793–807. doi: 10.1091/mbc.6.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoler S, Keith K C, Curnick K E, Fitzgerald-Hayes M. Genes Dev. 1995;9:573–586. doi: 10.1101/gad.9.5.573. [DOI] [PubMed] [Google Scholar]
- 26.Hyland K M, Kingsbury J, Koshland D, Hieter P. J Cell Biol. 1999;145:15–28. doi: 10.1083/jcb.145.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madeo F, Froehlich E, Ligr M, Grey M, Sigrist S J, Wolf D H, Froehlich K-U. J Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madeo F, Froehlich E, Froelich K-U. J Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenhalf W, Stephan C, Chaudhuri B. FEBS Lett. 1996;380:169–175. doi: 10.1016/0014-5793(96)00044-0. [DOI] [PubMed] [Google Scholar]
- 30.Zha H, Fisk H A, Yaffe M P, Mahajan N, Herman B, Reed J C. Mol Cell Biol. 1996;16:6494–6508. doi: 10.1128/mcb.16.11.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]